J Gastric Cancer.
2014 Mar;14(1):23-31.
Conventional Cytology Is Not Beneficial for Predicting Peritoneal Recurrence after Curative Surgery for Gastric Cancer: Results of a Prospective Clinical Study
- Affiliations
-
- 1Department of Surgery, Ajou University School of Medicine, Suwon, Korea. hhcmc75@naver.com
- 2Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
Abstract
- PURPOSE
The role of peritoneal washing cytology in determining further treatment strategies after surgery for gastric cancer remains unclear. One reason for this is the fact that optimal procedures to increase the accuracy of predicting peritoneal metastasis have not been established. The aim of this study was to evaluate the efficacy of cytology using samples harvested from two different abdominal cavity sites during gastric cancer surgery.
MATERIALS AND METHODS
We prospectively recruited 108 patients who were clinically diagnosed with locally advanced gastric cancer (higher than cT1 stage disease). Peritoneal washing fluids were collected from the pouch of Douglas and the subphrenic area. Patients were prospectively followed up for 2 years to determine the recurrence and survival rates.
RESULTS
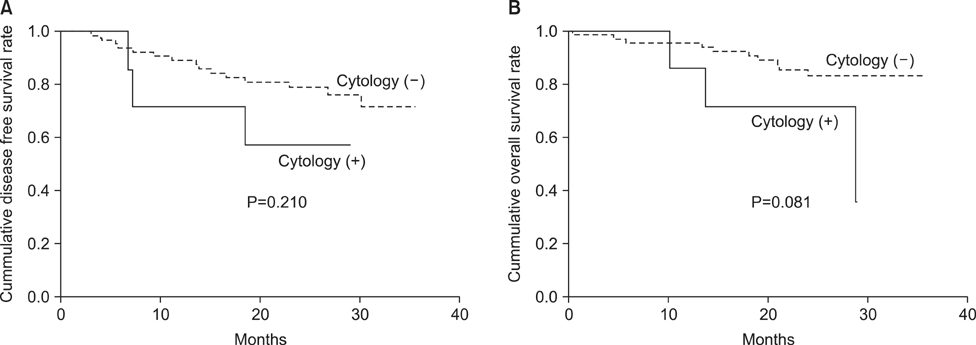
Thirty-three patients dropped out of the study for various reasons, so 75 patients were included in the final analysis. Seven patients (9.3%) showed positive cytology findings, of whom, three showed peritoneal recurrence. Tumor size was the only factor associated with positive cytology findings (P=0.037). The accuracy and specificity of cytology for predicting peritoneal recurrence were 90.1% and 94.2%, respectively, whereas the sensitivity was 50.0%. The survival rate did not differ between patients with positive cytology findings and those with negative cytology findings (P=0.081).
CONCLUSIONS
Peritoneal washing cytology using samples harvested from two different sites in the abdominal cavity was not able to predict peritoneal recurrence or survival in gastric cancer patients. Further studies will be required to determine whether peritoneal washing cytology during gastric cancer surgery is a meaningful procedure.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87:236–242.
Article3. Glockzin G, Piso P. Current status and future directions in gastric cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012; 21:625–633.
Article4. Matharu G, Tucker O, Alderson D. Systematic review of intraperitoneal chemotherapy for gastric cancer. Br J Surg. 2011; 98:1225–1235.
Article5. Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007; 14:2702–2713.
Article6. Kojima N, Kunieda K, Matsui K, Kato H, Saji S. Evaluation of carcinoembryonic antigen mRNA in living, necrotic, and apoptotic gastric cancer cells by reverse transcriptase-polymerase chain reaction. Surg Today. 2003; 33:839–846.
Article7. To EM, Chan WY, Chow C, Ng EK, Chung SC. Gastric cancer cell detection in peritoneal washing: cytology versus RT-PCR for CEA transcripts. Diagn Mol Pathol. 2003; 12:88–95.
Article8. Euanorasetr C, Lertsithichai P. Prognostic significance of peritoneal washing cytology in Thai patients with gastric adenocarcinoma undergoing curative D2 gastrectomy. Gastric Cancer. 2007; 10:18–23.
Article9. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999; 72:60–64. discussion 64-65.
Article10. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.11. Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumors. 7th ed. Hoboken: Wiley-Blackwell;2009.12. Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012; 15:Suppl 1. S27–S37.
Article13. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.14. Bernard N. Pleural, peritoneal and pericardial effusion. In : Bibbo M, editor. Comprehensive Cytopatholgy. 3rd ed. Philadelphia: Saunders;2008. p. 549–552.15. Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996; 83:672–674.
Article16. Abe S, Yoshimura H, Tabara H, Tachibana M, Monden N, Nakamura T, et al. Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol. 1995; 59:226–229.
Article17. Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990; 77:436–439.
Article18. Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994; 14:2131–2134.19. Sugita Y, Fujiwara Y, Taniguchi H, Mori T, Ishii T, Niwa H, et al. Quantitative molecular diagnosis of peritoneal lavage fluid for prediction of peritoneal recurrence in gastric cancer. Int J Oncol. 2003; 23:1419–1423.
Article20. Vogel P, Rüschoff J, Kümmel S, Zirngibl H, Hofstädter F, Hohenberger W, et al. Immunocytology improves prognostic impact of peritoneal tumour cell detection compared to conventional cytology in gastric cancer. Eur J Surg Oncol. 1999; 25:515–519.
Article21. Hayes N, Wayman J, Wadehra V, Scott DJ, Raimes SA, Griffin SM. Peritoneal cytology in the surgical evaluation of gastric carcinoma. Br J Cancer. 1999; 79:520–524.
Article22. Wu CC, Chen JT, Chang MC, Ho WL, Chen CY, Yeh DC, et al. Optimal surgical strategy for potentially curable serosa-involved gastric carcinoma with intraperitoneal free cancer cells. J Am Coll Surg. 1997; 184:611–617.23. de Manzoni G, Verlato G, Di Leo A, Tomezzoli A, Pedrazzani C, Pasini F, et al. Peritoneal cytology does not increase the prognostic information provided by TNM in gastric cancer. World J Surg. 2006; 30:579–584.
Article24. Fujii S, Kitayama J, Kaisaki S, Sasaki S, Seto Y, Tominaga O, et al. Carcinoembryonic antigen mRNA in abdominal cavity as a useful predictor of peritoneal recurrence of gastric cancer with serosal exposure. J Exp Clin Cancer Res. 2002; 21:547–553.25. Hara M, Nakanishi H, Jun Q, Kanemitsu Y, Ito S, Mochizuki Y, et al. Comparative analysis of intraperitoneal minimal free cancer cells between colorectal and gastric cancer patients using quantitative RT-PCR: possible reason for rare peritoneal recurrence in colorectal cancer. Clin Exp Metastasis. 2007; 24:179–189.
Article26. Yonemura Y, Fujimura T, Ninomiya I, Kim BS, Bandou E, Sawa T, et al. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res. 2001; 7:1647–1653.27. Tokuda K, Natsugoe S, Nakajo A, Miyazono F, Ishigami S, Hokita S, et al. Clinical significance of CEA-mRNA expression in peritoneal lavage fluid from patients with gastric cancer. Int J Mol Med. 2003; 11:79–84.
Article28. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321.
Article29. Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, et al. National Surgical Adjuvant Study of Gastric Cancer Group. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007; 94:1468–1476.
Article30. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820.
Article31. Roviello F, Marrelli D, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, et al. Italian Research Group for Gastric Cancer. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003; 90:1113–1119.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Free Cancer Cell Detection in Peritoneal Cavitr of Gastric Cancer Patients by RT-PCR for CEA
- Role of Peritoneal Lavage Cytology and Prediction of Prognosis and Peritoneal Recurrence After Curative Surgery for Colorectal Cancer
- Clinical Significance of Measuring Levels of CEA, CA19-9 in Peritoneal Washing Fluid in Patients with Gastric Cancer
- Clinicopathologic Characteristics according to the Type of Recurrence in Curatively-resected Gastric Cancer Patients
- The Result of Conversion Surgery in Gastric Cancer Patients with Peritoneal Seeding