J Gastric Cancer.
2011 Mar;11(1):16-22.
Eupatilin Inhibits Gastric Cancer Cell Growth by Blocking STAT3-Mediated VEGF Expression
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. jhcheong@yuhs.ac
- 2Seoul St. Mary's Hospital Convergent Research Consortium for Immunologic Disease, Seoul, Korea.
Abstract
- PURPOSE
Eupatilin is an antioxidative flavone and a phytopharmaceutical derived from Artemisia asiatica. It has been reported to possess anti-tumor activity in some types of cancer including gastric cancer. Eupatilin may modulate the angiogenesis pathway which is part of anti-inflammatory effect demonstrated in gastric mucosal injury models. Here we investigated the anti-tumor effects of eupatilin on gastric cancer cells and elucidated the potential underlying mechanism whereby eupatilin suppresses angiogenesis and tumor growth.
MATERIALS AND METHODS
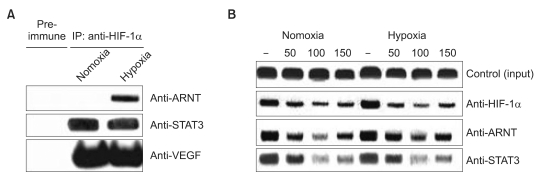
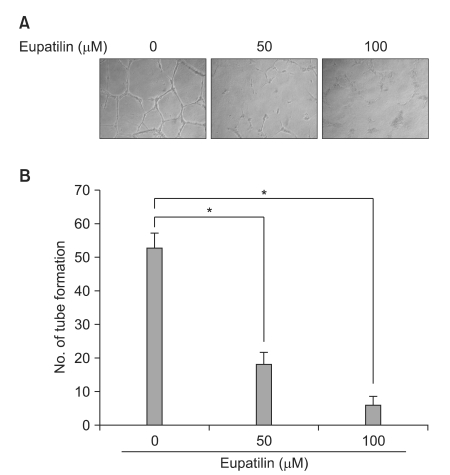
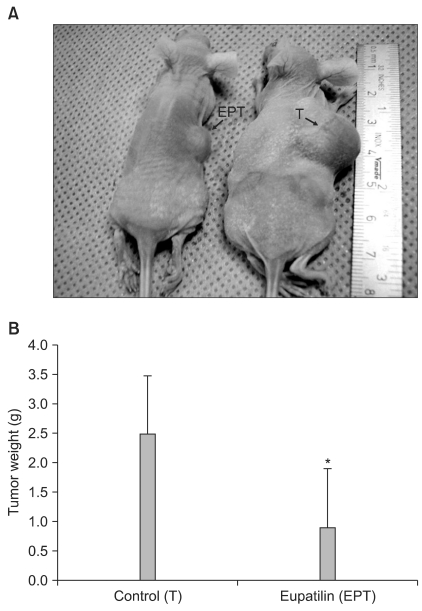
The impact of eupatilin on the expression of angiogenesis pathway proteins was assessed using western blots in MKN45 cells. Using a chromatin immunoprecipitation assay, we tested whether eupatilin affects the recruitment of signal transducer and activator of transcription 3 (STAT3), aryl hydrocarbon receptor nuclear translocator (ARNT) and hypoxia-inducible factor-1alpha (HIF-1alpha) to the human VEGF promoter. To investigate the effect of eupatilin on vasculogenesis, tube formation assays were conducted using human umbilical vein endothelial cells (HUVECs). The effect of eupatilin on tumor suppression in mouse xenografts was assessed.
RESULTS
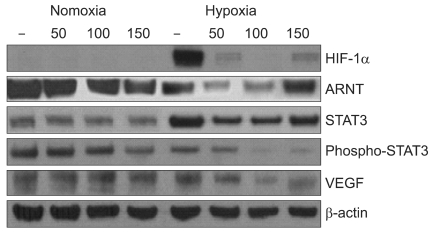
Eupatilin significantly reduced VEGF, ARNT and STAT3 expression prominently under hypoxic conditions. The recruitment of STAT3, ARNT and HIF-1alpha to the VEGF promoter was inhibited by eupatilin treatment. HUVECs produced much foreshortened and severely broken tubes with eupatilin treatment. In addition, eupatilin effectively reduced tumor growth in a mouse xenograft model.
CONCLUSIONS
Our results indicate that eupatilin inhibits angiogenesis in gastric cancer cells by blocking STAT3 and VEGF expression, suggesting its therapeutic potential in the treatment of gastric cancer.
MeSH Terms
-
Animals
Artemisia
Aryl Hydrocarbon Receptor Nuclear Translocator
Blotting, Western
Chromatin Immunoprecipitation
Flavones
Flavonoids
Human Umbilical Vein Endothelial Cells
Humans
Mice
Proteins
STAT3 Transcription Factor
Stomach Neoplasms
Transplantation, Heterologous
Vascular Endothelial Growth Factor A
Aryl Hydrocarbon Receptor Nuclear Translocator
Flavones
Flavonoids
Proteins
STAT3 Transcription Factor
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995; 333:1757–1763. PMID: 7491141.
Article2. Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999; 59:1592–1598. PMID: 10197634.3. Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Gliobalstoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994; 367:576–579. PMID: 8107827.4. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992; 359:843–845. PMID: 1279431.
Article5. Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000; 97:1749–1753. PMID: 10677529.
Article6. Rak J, Yu JL, Klement G, Kerbel RS. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J Investig Dermatol Symp Proc. 2000; 5:24–33.
Article7. Ellis LM, Staley CA, Liu W, Fleming RY, Parikh NU, Bucana CD, et al. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998; 273:1052–1057. PMID: 9422768.
Article8. Wiener JR, Nakano K, Kruzelock RP, Bucana CD, Bast RC Jr, Gallick GE. Decreased Src tyrosine kinase activity inhibits malignant human ovarian cancer tumor growth in a nude mouse model. Clin Cancer Res. 1999; 5:2164–2170. PMID: 10473101.9. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995; 92:5510–5514. PMID: 7539918.
Article10. Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999; 59:3915–3918. PMID: 10463582.11. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999; 15:551–578. PMID: 10611972.
Article12. Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000; 60:6189–6195. PMID: 11085544.13. Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000; 19:2474–2488. PMID: 10851046.
Article14. Catlett-Falcone R, Dalton WS, Jove R. STAT proteins as novel targets for cancer therapy. Signal transducer an activator of transcription. Curr Opin Oncol. 1999; 11:490–496. PMID: 10550013.16. Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000; 19:5419–5427. PMID: 11114718.
Article17. Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002; 21:2000–2008. PMID: 11960372.
Article18. Oh TY, Ryu BK, Ko JI, Ahn BO, Kim SH, Kim WB, et al. Protective effect of DA-9601, an extract of Artemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 1997; 20:414–419. PMID: 18982482.19. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, et al. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001; 49:364–371. PMID: 11511558.
Article20. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, et al. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001; 30:905–915. PMID: 11295533.
Article21. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285:1182–1186. PMID: 4938153.
Article22. Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001; 7:345–350. PMID: 11516994.
Article23. Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005; 24:3110–3120. PMID: 15735682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- STAT3, a Poor Survival Predicator, Is Associated with Lymph Node Metastasis from Breast Cancer
- Thrombospondin-1 and Inhibition of Tumor Growth
- Isoliquiritigenin Induces Apoptosis via ROS-Mediated Inhibition of p38/mTOR/STAT3 Pathway in Human Melanoma Cells
- Suppression of VEGF and STAT3 by Lipoic acid in Experimental Diabetic Rat Retina
- Endocrine Therapy Inhibits Expression of Vascular Endothelial Growth Factor and Angiogenesis in Prostate Cancer