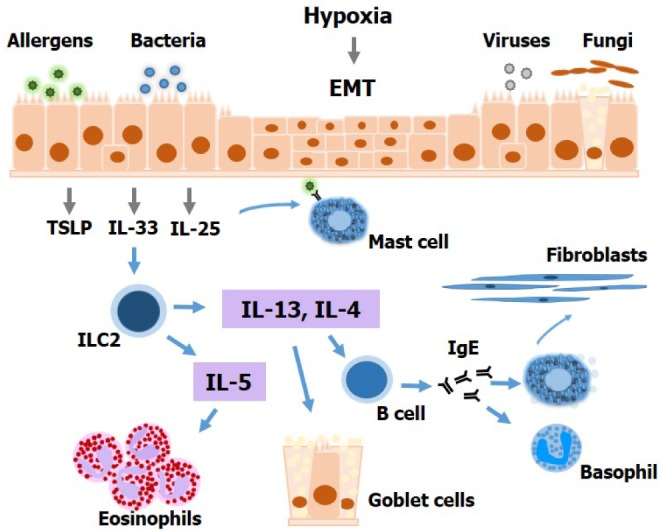
Immune Cell Responses and Mucosal Barrier Disruptions in Chronic Rhinosinusitis
- Affiliations
-
- 1Obstructive Upper airway Research (OUaR) Laboratory, Department of Pharmacology, Seoul National University College of Medicine, Seoul 03080, Korea. charlie@snu.ac.kr
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul 03080, Korea.
- KMID: 2368977
- DOI: http://doi.org/10.4110/in.2017.17.1.60
Abstract
- Chronic rhinosinusitis (CRS) is one of the most common presentations of upper airway illness and severely affects patient quality of life. Its frequency is not surprising given levels of environmental exposure to microbes, pollutants, and allergens. Inflammatory cells, inflammatory cytokine and chemokine production, and airway remodeling have been detected in the sinonasal mucosae of CRS patients, although the precise pathophysiological mechanisms causing such persistent inflammation remain unclear. Given its high prevalence and considerable associated morbidity, continued research into CRS is necessary to increase our understanding of factors likely to contribute to its pathogenesis, and facilitate the development of novel therapeutic strategies to improve treatment. The purpose of this review is to summarize the current state of knowledge regarding immune cell responses and epithelial alterations in CRS.
Keyword
MeSH Terms
Figure
Cited by 6 articles
-
Estrogen reinforces barrier formation and protects against tumor necrosis factor alpha-induced barrier dysfunction in oral epithelial cells
Yun Sik Choi, Keumjin Baek, Youngnim Choi
J Periodontal Implant Sci. 2018;48(5):284-294. doi: 10.5051/jpis.2018.48.5.284.Differential Hrd1 Expression and B-Cell Accumulation in Eosinophilic and Non-eosinophilic Chronic Rhinosinusitis With Nasal Polyps
Kun Chen, Miaomiao Han, Mengyao Tang, Yadong Xie, Yuting Lai, Xianting Hu, Jia Zhang, Jun Yang, Huabin Li
Allergy Asthma Immunol Res. 2018;10(6):698-715. doi: 10.4168/aair.2018.10.6.698.In-Depth, Proteomic Analysis of Nasal Secretions from Patients With Chronic Rhinosinusitis and Nasal Polyps
Yi-Sook Kim, Dohyun Han, JinYoup Kim, Dae Woo Kim, Yong-Min Kim, Ji-Hun Mo, Hyo-Geun Choi, Jong-Wan Park, Hyun-Woo Shin
Allergy Asthma Immunol Res. 2019;11(5):691-708. doi: 10.4168/aair.2019.11.5.691.Impact of the Long-Lived Plasma Cells in Patients With Chronic Rhinosinusitis With Nasal Polyps
Roza Khalmuratova, Hyun-Woo Shin
Allergy Asthma Immunol Res. 2020;12(2):173-175. doi: 10.4168/aair.2020.12.2.173.Evaluation of Neo-Osteogenesis in Eosinophilic Chronic Rhinosinusitis Using a Nasal Polyp Murine Model
Roza Khalmuratova, Mingyu Lee, Jong-Wan Park, Hyun-Woo Shin
Allergy Asthma Immunol Res. 2020;12(2):306-321. doi: 10.4168/aair.2020.12.2.306.Crosstalk Between Mucosal Inflammation and Bone Metabolism in Chronic Rhinosinusitis
Roza Khalmuratova, Hyun-Woo Shin
Clin Exp Otorhinolaryngol. 2021;14(1):43-49. doi: 10.21053/ceo.2020.00416.
Reference
-
1. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011; 128:693–707. PMID: 21890184.
Article2. Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. 2012; 26:110–116. PMID: 22487286.
Article3. Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol. 2015; 136:1442–1453. PMID: 26654193.
Article4. Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, Grammer LC, Schleimer RP. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008; 22:549–559. PMID: 18786300.
Article5. Payne SC, Borish L, Steinke JW. Genetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol. 2011; 128:710–720. PMID: 21704364.
Article6. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, Wang DY, Desrosiers M, Liu Z. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; 124:478–484. 484.e1–484.e2. PMID: 19541359.
Article7. Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007; 137:925–930. PMID: 18036422.
Article8. Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, Saitoh T, Murata J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013; 123:E1–E9. PMID: 23670893.
Article9. Wen W, Liu W, Zhang L, Bai J, Fan Y, Xia W, Luo Q, Zheng J, Wang H, Li Z, Xia J, Jiang H, Liu Z, Shi J, Li H, Xu G. Nasal Health Group, China (NHGC). Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012; 129:1522–1528. PMID: 22460066.
Article10. Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013; 149:431–437. PMID: 23812744.
Article11. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289. PMID: 17002703.
Article12. Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, Gevaert P. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008; 121:1435–1441. PMID: 18423831.
Article13. Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, Jung JY, Hwang GH, Lee SH. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol. 2013; 131:772–780. PMID: 23375208.
Article14. Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, Suh LA, Norton J, Harris KE, Grammer LC, Chandra RK, Conley DB, Kern RC, Schleimer RP, Kato A. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013; 132:593–600. PMID: 23688414.
Article15. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–968. PMID: 18804271.
Article16. Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, Cuvelier C, Van Cauwenberge P, Bachert C. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124:253–259. PMID: 19500825.17. Sejima T, Holtappels G, Kikuchi H, Imayoshi S, Ichimura K, Bachert C. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int. 2012; 61:115–122. PMID: 22377524.
Article18. Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, Cui YH, Liu Z. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013; 68:101–109. PMID: 23157215.
Article19. Van Bruaene N, C PN, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, Gevaert P, Bachert C. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy. 2012; 42:883–890. PMID: 22093003.
Article20. Bachert C, Van Bruaene N, Toskala E, Zhang N, Olze H, Scadding G, Van Drunen CM, Mullol J, Cardell L, Gevaert P, Van Zele T, Claeys S, Hallden C, Kostamo K, Foerster U, Kowalski M, Bieniek K, Olszewska-Ziaber A, Nizankowska-Mogilnicka E, Szczeklik A, Swierczynska M, Arcimowicz M, Lund V, Fokkens W, Zuberbier T, Akdis C, Canonica G, Van Cauwenberge P, Burney P, Bousquet J. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyposis - a GALEN study. Allergy. 2009; 64:520–533. PMID: 19317839.21. Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006; 61:1275–1279. PMID: 17002702.
Article22. Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015; 64:121–130. PMID: 25838086.
Article23. Katotomichelakis M, Tantilipikorn P, Holtappels G, De Ruyck N, Feng L, Van Zele T, Muangsomboon S, Jareonchasri P, Bunnag C, Danielides V, Cuvelier CA, Hellings PW, Bachert C, Zhang N. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013; 27:354–360. PMID: 23816657.
Article24. Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res. 2010; 2:87–101. PMID: 20358022.
Article25. Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009; 21:1303–1309. PMID: 19819937.
Article26. Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997; 99:837–842. PMID: 9215253.27. Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001; 2:95–101. PMID: 11175800.
Article28. Yao T, Kojima Y, Koyanagi A, Yokoi H, Saito T, Kawano K, Furukawa M, Kusunoki T, Ikeda K. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009; 119:1053–1059. PMID: 19296494.
Article29. Olze H, Forster U, Zuberbier T, Morawietz L, Luger EO. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006; 44:145–150. PMID: 16792175.30. Early SB, Hise K, Han JK, Borish L, Steinke JW. Hypoxia stimulates inflammatory and fibrotic responses from nasal-polyp derived fibroblasts. Laryngoscope. 2007; 117:511–515. PMID: 17334314.
Article31. Nissim Ben Efraim AH, Eliashar R, Levi-Schaffer F. Hypoxia modulates human eosinophil function. Clin Mol Allergy. 2010; 8:10. PMID: 20642833.
Article32. Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, Grammer LC, Tan BK, Chandra RK, Conley DB, Kern RC, Fujieda S, Schleimer RP. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012; 130:410–420. PMID: 22534535.
Article33. Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, Zeng M, Liu WH, Schleimer RP, Liu Z. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014; 44:690–700. PMID: 24597471.
Article34. Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, Kern RC, Conley DB, Chandra R, Hulse KE, Suh LA, Norton JE, Peters AT, Grammer LC, Schwartz LB, Schleimer RP. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014; 133:1759–1763. PMID: 24636088.
Article35. Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012; 130:1087–1096. PMID: 22840853.36. Nomura K, Obata K, Keira T, Miyata R, Hirakawa S, Takano K, Kohno T, Sawada N, Himi T, Kojima T. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir Res. 2014; 15:21. PMID: 24548792.
Article37. Shin HW, Cho K, Kim DW, Han DH, Khalmuratova R, Kim SW, Jeon SY, Min YG, Lee CH, Rhee CS, Park JW. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med. 2012; 185:944–954. PMID: 22323302.
Article38. Hupin C, Gohy S, Bouzin C, Lecocq M, Polette M, Pilette C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy. 2014; 69:1540–1549. PMID: 25104359.
Article39. Lee M, Kim DW, Yoon H, So D, Khalmuratova R, Rhee CS, Park JW, Shin HW. Sirtuin 1 attenuates nasal polypogenesis by suppressing epithelial-to-mesenchymal transition. J Allergy Clin Immunol. 2016; 137:87–98. PMID: 26342525.
Article40. Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010; 38:864–878. PMID: 20620956.41. Yoon H, Shin SH, Shin DH, Chun YS, Park JW. Differential roles of Sirt1 in HIF-1alpha and HIF-2alpha mediated hypoxic responses. Biochem Biophys Res Commun. 2014; 444:36–43. PMID: 24423936.42. Kim SW, Kim DW, Khalmuratova R, Kim JH, Jung MH, Chang DY, Shin EC, Lee HK, Shin HW, Rhee CS, Jeon SY, Min YG. Resveratrol prevents development of eosinophilic rhinosinusitis with nasal polyps in a mouse model. Allergy. 2013; 68:862–869. PMID: 23751068.
Article43. Zhang N, Van Crombruggen K, Gevaert E, Bachert C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016; 71:295–307. PMID: 26606240.
Article44. Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O'Connor W Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011; 475:514–518. PMID: 21765430.
Article45. Ke Y, Liu K, Huang GQ, Cui Y, Kaplan HJ, Shao H, Sun D. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009; 182:3183–3190. PMID: 19234216.
Article46. Otani K, Watanabe T, Tanigawa T, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Arakawa T. Anti-inflammatory effects of IL-17A on Helicobacter pylori-induced gastritis. Biochem Biophys Res Commun. 2009; 382:252–258. PMID: 19249291.
Article47. Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011; 134:8–16. PMID: 21726218.
Article48. Zizzo G, Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. J Immunol. 2013; 190:5237–5246. PMID: 23596310.
Article49. Lee M, Kim DW, Shin HW. Targeting IL-25 as a novel therapy in chronic rhinosinusitis with nasal polyps. Curr Opin Allergy Clin Immunol. 2017; 17:17–22. PMID: 27870664.
Article50. Pappu R, Rutz S, Ouyang W. Regulation of epithelial immunity by IL-17 family cytokines. Trends Immunol. 2012; 33:343–349. PMID: 22476048.
Article51. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002; 169:443–453. PMID: 12077275.
Article52. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001; 15:985–995. PMID: 11754819.53. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007; 120:1324–1331. PMID: 17889290.
Article54. Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, Kong IG, Mo JH, Yang MS, Jin HR, Park JW, Kim DW. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015; 135:1476–1485. PMID: 25725991.
Article55. Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, Vreugde S. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014; 69:1154–1161. PMID: 24924975.
Article56. Mjosberg JM, Trifari S, Crellin NK, Peters CP, Van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12:1055–1062. PMID: 21909091.
Article57. Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010; 40:200–208. PMID: 19906013.
Article58. Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010; 88:257–268. PMID: 20065993.
Article59. Lam M, Hull L, McLachlan R, Snidvongs K, Chin D, Pratt E, Kalish L, Sacks R, Earls P, Sewell W, Harvey RJ. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3:121–128. PMID: 23038685.
Article60. Kim DK, Jin HR, Eun KM, Mo JH, Cho SH, Oh S, Cho D, Kim DW. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2016; DOI: 10.1136/thoraxjnl-2016-208772.
Article61. Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM, Delespesse G. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009; 123:472–478. PMID: 19064280.62. Kim DW, Eun KM, Jin HR, Cho SH, Kim DK. Prolonged allergen exposure is associated with increased thymic stromal lymphopoietin expression and Th2-skewing in mouse models of chronic rhinosinusitis. Laryngoscope. 2016; 126:E265–E272. PMID: 27107152.
Article63. Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, Lee CH, Rhee CS. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011; 25:e255–e261. PMID: 22185735.
Article64. Khalmuratova R, Lee M, Kim DW, Park JW, Shin HW. Induction of nasal polyps using house dust mite and Staphylococcal enterotoxin B in C57BL/6 mice. Allergol Immunopathol(Madr.). 2016; 44:66–75. PMID: 26242569.
Article65. Shin HW. Animal models in CRS and pathophysiologic insights gained: A systematic review. Laryngoscope Investig Otolaryngol. 2016; 1:116–123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Innate Immune Responses in Pathogenesis of Chronic Rhinosinusitis
- Pathogenesis of Recalcitrant Chronic Rhinosinusitis: The Emerging Role of Innate Immune Cells
- The Role of Epithelium in the Pathophysiology of Chronic Rhinosinusitis : An Update
- Mechanisms of Glucocorticoid Action in Chronic Rhinosinusitis
- Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors