Nat Prod Sci.
2016 Dec;22(4):252-258. 10.20307/nps.2016.22.4.252.
Inhibition of Melanogenesis by Abietatriene from Vitex Trifolia Leaf Oil
- Affiliations
-
- 1Research & Development Center, LG Household & Healthcare Ltd.; 175, Gajeong-ro, Youseong-gu, Daejeon 305-343, South Korea. mhjin@lgcare.com
- 2Herbal Experiment Station, Jeonbuk A.R.E.S. Medicinal Resources Research Institute.; 108, Haengjeonggongan-gil, Unbong-eup, Namwon-si, Jeollabuk-do, Korea.
- KMID: 2366692
- DOI: http://doi.org/10.20307/nps.2016.22.4.252
Abstract
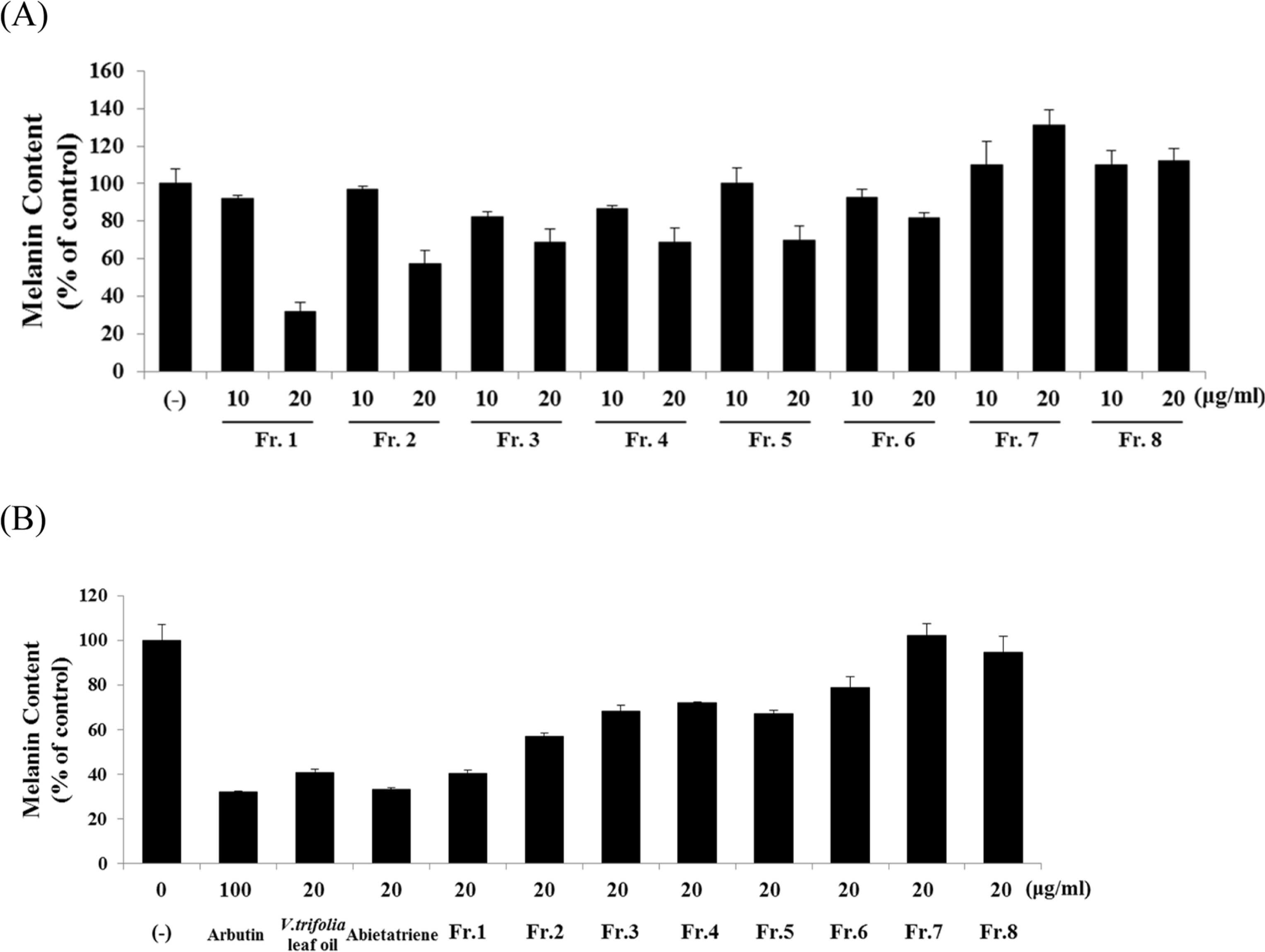
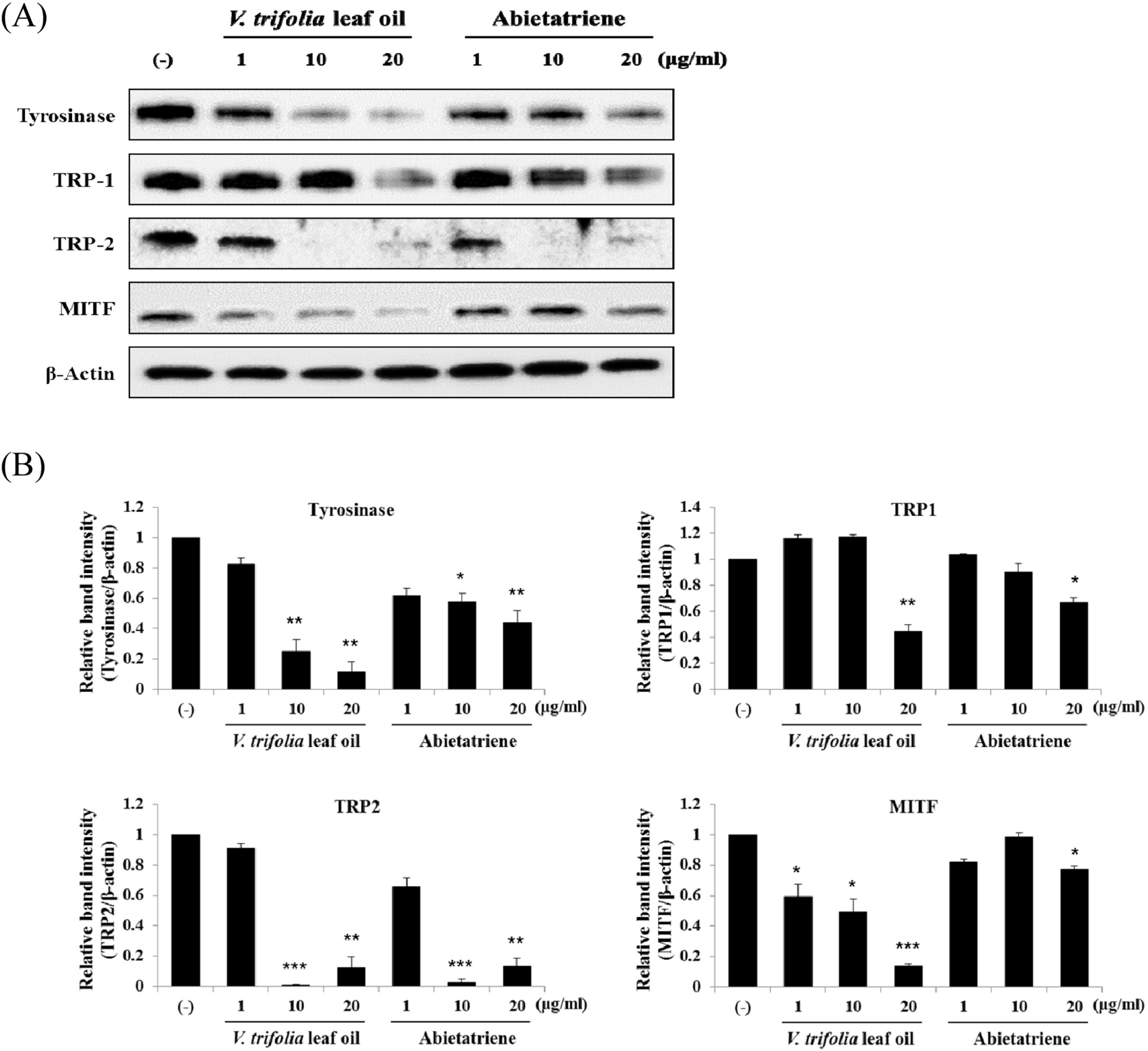
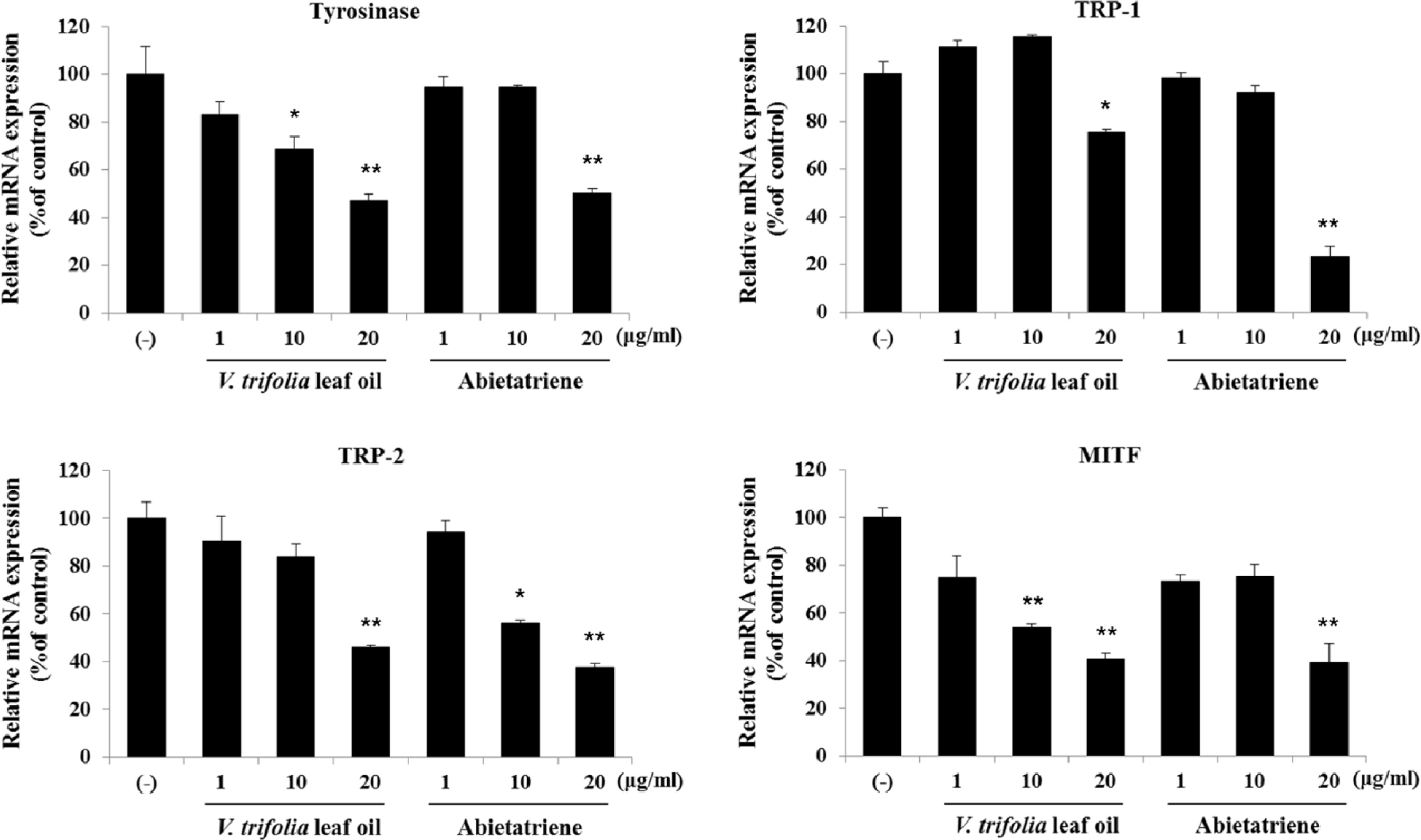
- Vitex trifolia L. has been used traditionally to treat various illnesses, such as inflammation, headache, migraine, and gastrointestinal infections. We analyzed and evaluated the composition of V. trifolia leaf oil. Based on the results, we isolated abietatriene from V. trifolia leaf oil and investigated the effect of V. trifolia leaf oil and its active compound abietatriene on melanogenesis in B16F10 melanoma cells. They significantly decreased melanin contents and melanogenic factors, such as tyrosinase, TRP-1, TRP-2, and MITF dose-dependently in both protein and mRNA levels. Protein and mRNA expressions were determined by Western blot analysis and quantitative real time RT-PCR. Findings indicate that V. trifolia leaf oil and abietatriene reduce melanogenesis by regulating the expression of melanogenic factors. These results suggest that V. trifolia leaf oil and abietatriene could comprise a useful therapeutic agent for treating hyperpigmentation and used as effective skin-whitening agents.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Costin G. E.., Hearing V. J.FASEB J. 2007. 21:976–994.(2). Lin J.Y.., Fisher D. E.Nature. 2007. 445:843–850.(3). del Marmol V.., Beermann F.FEBS let. 1996. 381:165–168.
Article(4). Tsukamoto K.., Jackson I. J.., Urabe K.., Montague P. M.., Hearing V. J.EMBO J. 1992. 11:519–526.(5). Bentley N. J.., Eisen T.., Goding C. R.Mol. Cell. Biol. 1994. 14:7996–8006.(6). Yasumoto K.., Yokoyama K.., Takahashi K.., Tomita Y.., Shibahara S. J.Biol. Chem. 1997. 272:503–509.(7). Sugumaran M.Pigment Cell Res. 2002. 15:2–9.(8). Chen J. S.., Wei C. I.., Marshall M. R. J.Agric. Food Chem. 1991. 39:1897–1901.(9). Zhu W.., Gao J. J.Investig. Dermatol. Symp. Proc. 2008. 13:20–24.(10). Kimura T.., Kimura T.Medicinal Plants of Japan in Color; Hoikusha Publishing: Osaka,. 1981. , p. 183.(11). Ono M.., Sawamura H.., Ito Y.., Mizuki K.., Nohara T.Phytochemistry. 2000. 8:873–877.(12). Park J. H.., Lee C. K.The Encyclopedia of Medicinal Plants; Shinilbooks: Korea,. 2000. 183–184:284–289.(13). Kim C. M.., Lee Y. J.., Kim I. L.., Shin J. H.., Kim Y. I.Coloured Illustrations dor Discrimination of Herbal Medicine; Academybooks: Korea:. 2015. , p. 278.(14). Huang M. Y.., Zhong L. J.., Xie J. M.., Wang F.., Zhang H. Y.Helv. Chim. Acta. 2013. 96:2040–2045.(15). Institute of Materia Medica. Chinese Academy of Medical Sciences, Chinese Materia Medica. People's Medical Publishing House;Beijing: 1984. Vol. 3:, p. p. 679.(16). Lee M. K.., Kim D. H.., Park T. S.., Son J. H. J.Appl. Biol. Chem. 2015. 58:125–129.(17). Ramesh P.., Nair A. G. R.., Subramanian S. S.Fitoterapia. 1986. LVII(4):282–283.(18). Thein K.., Myint W.., Myint M. M.., Aung S. P.., Khin M.., Than A.., Bwin M.Pharm. Biol. 1995. 33:330–333.(19). Hernández M. M.., Heraso C.., Villarreal M. L.., Vargas-Arispuro I.., Aranda E. J.Ethnopharmacol. 1999. 67:37–44.(20). Hossain M. M.., Paul N.., Sohrab M. H.., Rahman E.., Rashid M. A.Fitoterapia. 2001. 72:695–697.(21). Zeng X.., Fang Z.., Wu Y.., Zhang H.Chung Kuo Chung Yao Tsa Chih. 1996. 21:167–168.(22). Ramesh P.., Nair A. G. R.Fitoterapia. 1986. 57:282.(23). Nair A. G. R.., Ramesh P.., Subramanian S. S.Curr. Sci. 1975. 44:214–216.(24). Vedantham T. N. C.., Subramanian S. S.Indian J. Pharmacol. 1976. 38:13–24.(25). Pan J. G.., Xu Z. L.., Fan J. F.Chung Kuo Chung Yao Tsa Chih. 1989.(26). Zai-bo Y.., Chao Z.Journal of Henan University(Medical Science). 2006. 4:4.(27). Suksamrarn A.., Werawattanametin K.., Brophy J. J.Flavour Frag. J. 1991. 6:97–99.(28). Hill S. E.., Buffey J.., Thody A. J.., Oliver I.., Bleehen S. S.., Mac Neil S.Pigment Cell Res. 1989. 2:161–166.(29). Livak K. J.., Schmittgen T. D.Methods. 2001. 25:402–408.(30). Zi J.., Peters R. J.Org. Biomol. Chem. 2013. 11:7650–7652.(31). Bhar S. S.., Ramana M. M. V. J.Org. Chem. 2004. 69:8935–8937.(32). Yamaguchi Y.., Beer J. Z.., Hearing V. J.Arch. Dermatol. Res. 2008. 300:43–50.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quality Characteristics of Rice Cookies Prepared with Stevia rebaudiana Leaf
- Antibacterial Effects of Tea Tree Oil and Mastic Oil to Streptococcus mutans
- Comparison of Vitex agnus-castus Extracts with Placebo in Reducing Menopausal Symptoms: A Randomized Double-Blind Study
- Chemical Investigation on an Endophytic fungus Gibberella moniliformis JS1055 Derived from a Halophyte Vitex rotundifolia
- Comparative evaluation of pain perception following topical application of clove oil, betel leaf extract, lignocaine gel, and ice prior to intraoral injection in children aged 6-10 years: a randomized control study