Yonsei Med J.
2015 Jul;56(4):1106-1113. 10.3349/ymj.2015.56.4.1106.
Fracture Healing Effects of Locally-Administered Adipose Tissue-Derived Cells
- Affiliations
-
- 1Department of Radiology, Gil Hospital, Gachon University School of Medicine and Science, Incheon, Korea.
- 2Department of Nuclear Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. tjeonnm@yuhs.ac
- 3Department of Radiology, Stanford University School of Medicine, Stanford, CA, USA.
- KMID: 2366354
- DOI: http://doi.org/10.3349/ymj.2015.56.4.1106
Abstract
- PURPOSE
Although the applications of adipose tissue-derived cells (ADCs) in regenerative medicine have been investigated, the role of ADCs in fracture healing remains unclear. In this study, we examined the fracture-healing effects and survival of transplanted ADCs using micro-computed tomography (CT) and bioluminescence imaging (BLI).
MATERIALS AND METHODS
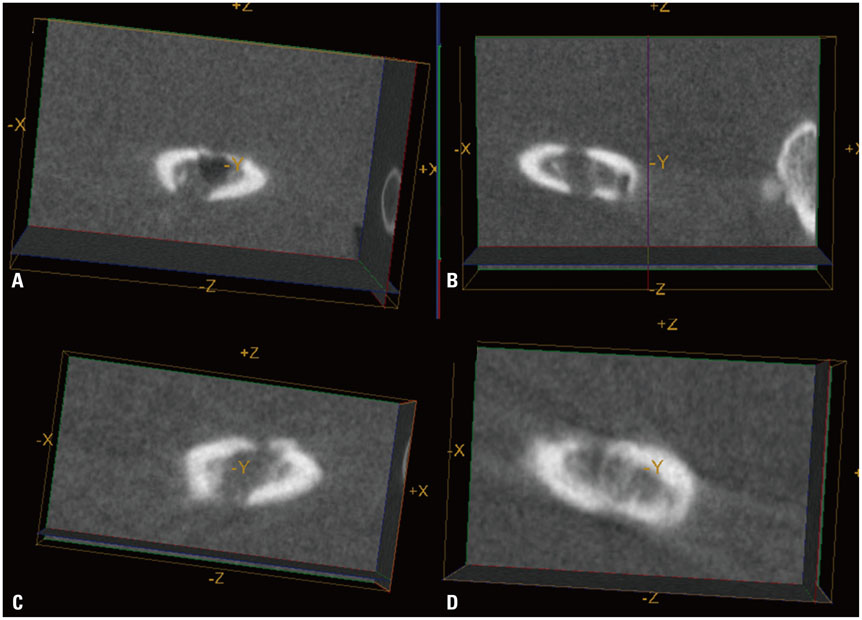
Luciferase-expressing ADCs were suspended in solubilized basement membrane preparation (SBMP) and xenografted on defects in the right femur of nude mice (n=5). SBMP alone was grafted on a defect in the contralateral femur. Serial in vivo micro-CT and BLI were performed for 20 days. Ex vivo BLI images of both femurs were obtained. Differences in the Hounsfield unit (HU), HUratio, and luciferase activities were compared using Wilcoxon signed-rank tests and non-parametric longitudinal analyses (p<0.05).
RESULTS
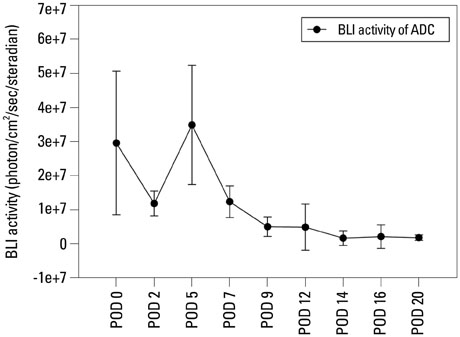
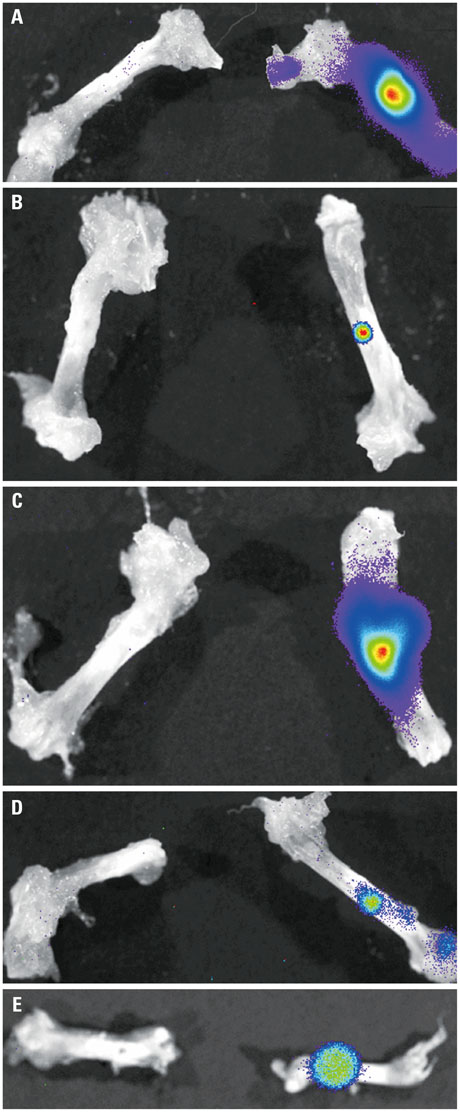
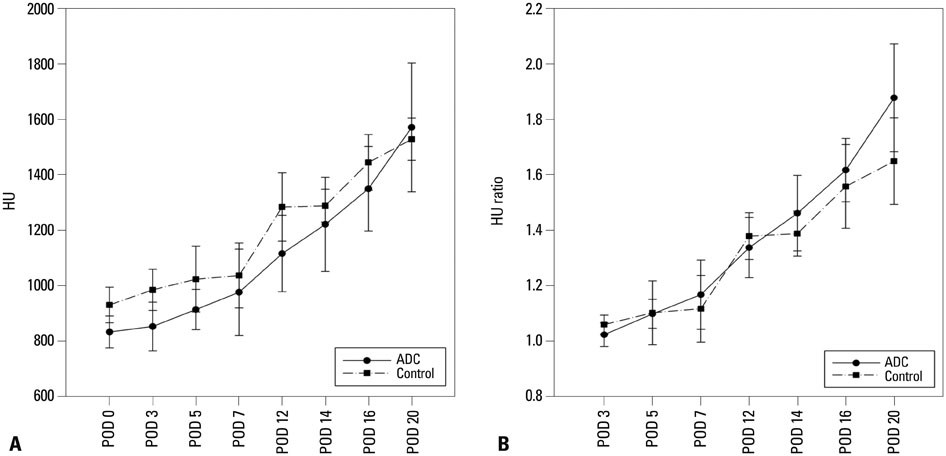
In vivo BLI revealed a signal drop on day 2, reconstitution on day 5, and continuous decrement thereafter. Ex vivo BLI revealed residual activity in the ADC-implanted and adjacent areas. No activity was detected in the contralateral femur. The overall increment rate of normalized HUs was higher for ADC-treated femurs than for SBMP-treated femurs. Cell migration to distant injury sites was not detected.
CONCLUSION
Enhanced bone density in the implant area suggests that ADCs have fracture-healing effects.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The effects of pentoxifylline adminstration on fracture healing in a postmenopausal osteoporotic rat model
Mohammad Mahdi Vashghani Farahani, Reza Ahadi, Mohammadamin Abdollahifar, Mohammad Bayat
Lab Anim Res. 2017;33(1):15-23. doi: 10.5625/lar.2017.33.1.15.
Reference
-
1. Brighton CT, Shaman P, Heppenstall RB, Esterhai JL Jr, Pollack SR, Friedenberg ZB. Tibial nonunion treated with direct current, capacitive coupling, or bone graft. Clin Orthop Relat Res. 1995; 223–234.
Article2. Giannoudis P, Psarakis S, Kontakis G. Can we accelerate fracture healing? A critical analysis of the literature. Injury. 2007; 38:Suppl 1. S81–S89.3. Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules. Injury. 2007; 38:Suppl 1. S11–S25.4. Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007; 38:Suppl 1. S26–S32.
Article5. Knight MN, Hankenson KD. Mesenchymal Stem Cells in Bone Regeneration. Adv Wound Care (New Rochelle). 2013; 2:306–316.
Article6. De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001; 44:1928–1942.
Article7. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295.
Article8. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301.
Article9. Shoji T, Ii M, Mifune Y, Matsumoto T, Kawamoto A, Kwon SM, et al. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest. 2010; 90:637–649.
Article10. Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005; 11:120–129.
Article11. De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003; 174:101–109.
Article12. Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998; 355 Suppl. S247–S256.
Article13. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004; 22:560–567.
Article14. Lee SW, Padmanabhan P, Ray P, Gambhir SS, Doyle T, Contag C, et al. Stem cell-mediated accelerated bone healing observed with in vivo molecular and small animal imaging technologies in a model of skeletal injury. J Orthop Res. 2009; 27:295–302.
Article15. Li H, Dai K, Tang T, Zhang X, Yan M, Lou J. Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun. 2007; 356:836–842.
Article16. Bigham-Sadegh A, Oryan A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int Wound J. 2014; 02. 21. [Epub]. http://dx.doi.org/10.1111/iwj.12231.
Article17. Schubert T, Lafont S, Beaurin G, Grisay G, Behets C, Gianello P, et al. Critical size bone defect reconstruction by an autologous 3D osteogenic-like tissue derived from differentiated adipose MSCs. Biomaterials. 2013; 34:4428–4438.
Article18. Schubert T, Xhema D, Vériter S, Schubert M, Behets C, Delloye C, et al. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials. 2011; 32:8880–8891.
Article19. Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998; 16:155–162.
Article20. Lyons FG, Al-Munajjed AA, Kieran SM, Toner ME, Murphy CM, Duffy GP, et al. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010; 31:9232–9243.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- Clinical Application of Adipose Derived Stromal Cell Autograft for Wound Coverage
- Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing
- Adipose-derived stem cells: characterization and clinical application
- Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing