Korean J Pain.
2016 Apr;29(2):86-95. 10.3344/kjp.2016.29.2.86.
Comparison of Mechanical Allodynia and Recovery of Locomotion and Bladder Function by Different Parameters of Low Thoracic Spinal Contusion Injury in Rats
- Affiliations
-
- 1Department of Neuroscience and Cell Biology, University of Texas Medical Branch at Galveston, TX, USA.
- 2Department of Physiology, Daegu Haany University, Daegu, Korea. ysgwak@dhu.ac.kr
- KMID: 2365350
- DOI: http://doi.org/10.3344/kjp.2016.29.2.86
Abstract
- BACKGROUND
The present study was designed to examine the functional recovery following spinal cord injury (SCI) by adjusting the parameters of impact force and dwell-time using the Infinite Horizon (IH) impactor device.
METHODS
Sprague-Dawley rats (225-240 g) were divided into eight injury groups based on force of injury (Kdyn) and dwell time (seconds), indicated as Force-Dwell time: 150-4, 150-3, 150-2, 150-1, 150-0, 200-0, 90-2 and sham controls, respectively.
RESULTS
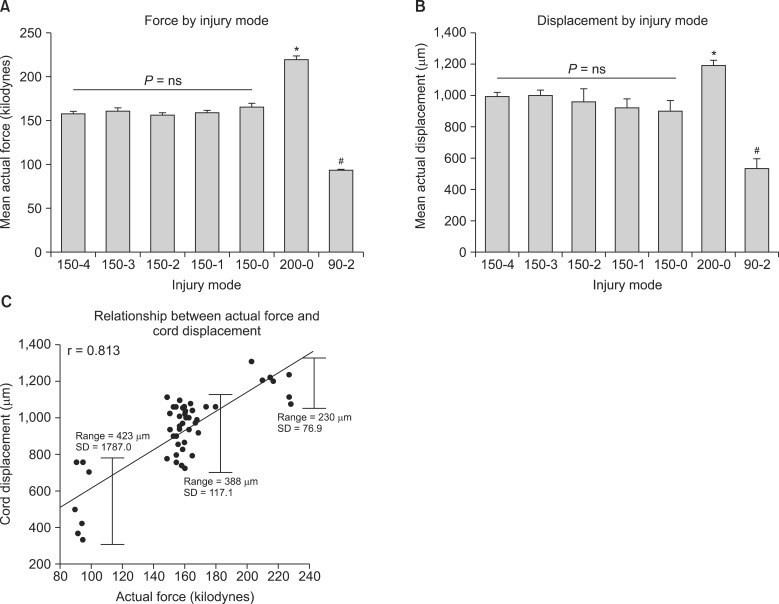
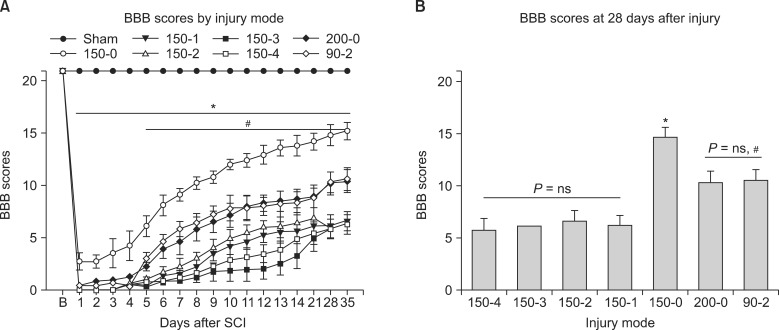
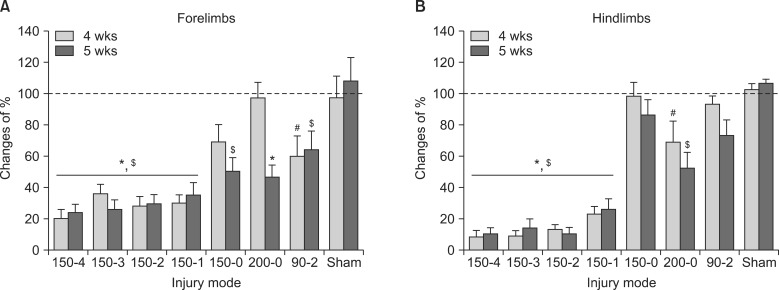
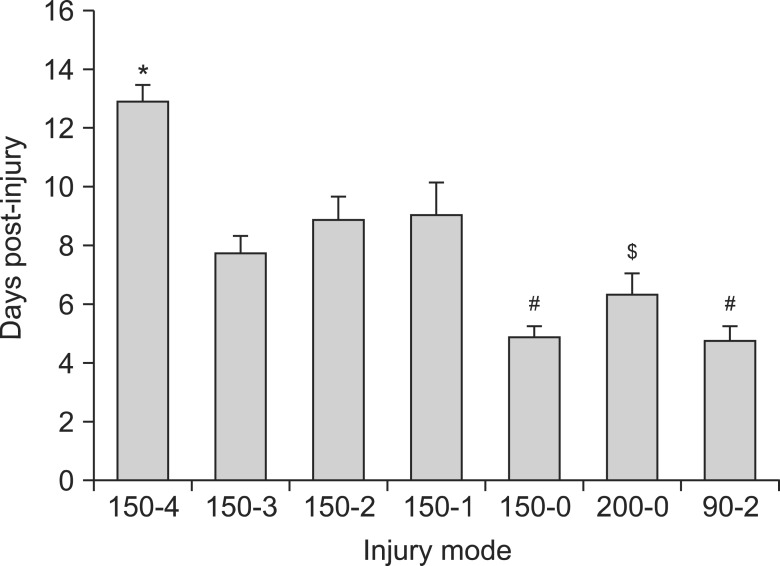
After T10 SCI, higher injury force produced greater spinal cord displacement (P < 0.05) and showed a significant correlation (r = 0.813) between the displacement and the force (P < 0.05). In neuropathic pain-like behavior, the percent of paw withdrawals scores in the hindpaw for the 150-4, 150-3, 150-2, 150-1 and the 200-0 injury groups were significantly lowered compared with sham controls (P < 0.05). The recovery of locomotion had a significant within-subjects effect of time (P < 0.05) and the 150-0 group had increased recovery compared to other groups (P < 0.05). In addition, the 200-0 and the 90-2 recovered significantly better than all the 150 kdyn impact groups that included a dwell-time (P < 0.05). In recovery of spontaneous bladder function, the 150-4 injury group took significantly longer recovery time whereas the 150-0 and the 90-2 groups had the shortest recovery times.
CONCLUSIONS
The present study demonstrates SCI parameters optimize development of mechanical allodynia and other pathological outcomes.
Keyword
MeSH Terms
Figure
Reference
-
1. Bruce JC, Oatway MA, Weaver LC. Chronic pain after clip-compression injury of the rat spinal cord. Exp Neurol. 2002; 178:33–48. PMID: 12460606.
Article2. Mills CD, Grady JJ, Hulsebosch CE. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J Neurotrauma. 2001; 18:1091–1105. PMID: 11686495.
Article3. Vierck CJ Jr, Light AR. Allodynia and hyperalgesia within dermatomes caudal to a spinal cord injury in primates and rodents. Prog Brain Res. 2000; 129:411–428. PMID: 11098708.
Article4. Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair. 2000; 14:287–300. PMID: 11402879.
Article5. Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000; 17:1205–1217. PMID: 11186233.
Article6. Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998; 75:141–155. PMID: 9539683.
Article7. Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996; 68:97–107. PMID: 9252004.
Article8. Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport. 1995; 6:1241–1244. PMID: 7669978.
Article9. Ovelmen-Levitt J, Gorecki J, Nguyen KT, Iskandar B, Nashold BS Jr. Spontaneous and evoked dysesthesias observed in the rat after spinal cordotomies. Stereotact Funct Neurosurg. 1995; 65:157–160. PMID: 8916347.
Article10. Xu XJ, Hao JX, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992; 48:279–290. PMID: 1589248.
Article11. Hao JX, Xu XJ, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Allodynia-like effects in rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain. 1991; 45:175–185. PMID: 1652116.
Article12. Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993; 59:75–89. PMID: 8420126.13. Kakulas BA, Taylor JR. Pathology of injuries of the vertebral column and spinal cord. In : Vinken PJ, Bruyn GW, Klawans HL, Frankel HL, editors. Handbook of clinical neurology. Vol 17. Amsterdam: Elsevier;1992. p. 21–51.14. Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000; 17:1–17. PMID: 10674754.
Article15. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996; 139:244–256. PMID: 8654527.
Article16. Constantini S, Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg. 1994; 80:97–111. PMID: 8271028.
Article17. Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992; 9:123–126. PMID: 1404425.
Article18. Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985; 88:108–122. PMID: 3979505.
Article19. Stokes BT, Noyes DH, Behrmann DL. An electromechanical spinal injury technique with dynamic sensitivity. J Neurotrauma. 1992; 9:187–195. PMID: 1474607.
Article20. Stokes BT. Experimental spinal cord injury: a dynamic and verifiable injury device. J Neurotrauma. 1992; 9:129–131. PMID: 1404426.
Article21. Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003; 20:179–193. PMID: 12675971.
Article22. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21. PMID: 7783230.
Article23. Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci. 2001; 21:559–569. PMID: 11160435.
Article24. Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997; 14:517–537. PMID: 9300563.
Article25. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994; 59:369–376. PMID: 7708411.
Article26. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363. PMID: 1333581.
Article27. Wrathall JR, Emch GS. Effect of injury severity on lower urinary tract function after experimental spinal cord injury. Prog Brain Res. 2006; 152:117–134. PMID: 16198697.
Article28. Streijger F, Beernink TM, Lee JH, Bhatnagar T, Park S, Kwon BK, et al. Characterization of a cervical spinal cord hemicontusion injury in mice using the infinite horizon impactor. J Neurotrauma. 2013; 30:869–883. PMID: 23360150.
Article29. Kato H, Kanellopoulos GK, Matsuo S, Wu YJ, Jacquin MF, Hsu CY, et al. Neuronal apoptosis and necrosis following spinal cord ischemia in the rat. Exp Neurol. 1997; 148:464–474. PMID: 9417826.
Article30. Rokkas CK, Cronin CS, Nitta T, Helfrich LR Jr, Lobner DC, Choi DW, et al. Profound systemic hypothermia inhibits the release of neurotransmitter amino acids in spinal cord ischemia. J Thorac Cardiovasc Surg. 1995; 110:27–35. PMID: 7609553.
Article31. Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996; 27:1850–1858. PMID: 8841344.
Article32. Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978; 10:38–43. PMID: 684604.33. Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004; 21:1371–1383. PMID: 15672628.
Article34. Ward PJ, Hubscher CH. Persistent polyuria in a rat spinal contusion model. J Neurotrauma. 2012; 29:2490–2498. PMID: 22708983.
Article35. Sharp K, Yee KM, Steward O. A re-assessment of the effects of treatment with an epidermal growth factor receptor (EGFR) inhibitor on recovery of bladder and locomotor function following thoracic spinal cord injury in rats. Exp Neurol. 2012; 233:649–659. PMID: 22078761.
Article36. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006; 152:59–84. PMID: 16198694.
Article37. David BT, Steward O. Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp Neurol. 2010; 226:128–135. PMID: 20713043.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animals models of spinal cord contusion injury
- Long-term Follow-up of Cutaneous Hypersensitivity in Rats with a Spinal Cord Contusion
- Effect of Cyclosporin A in a Rat Spinal Cord Injury Model
- Clinical Features of Central Neuropathic Pain after Contusive Spinal Cord Injury in Rats
- Causalgiform pain produced by the tight ligation of L5, L6 spinal nerves in the rat