Korean Circ J.
2017 Jan;47(1):115-122. 10.4070/kcj.2016.0278.
Effect of Stents Coated with Artemisinin or Dihydroartemisinin in a Porcine Coronary Restenosis Model
- Affiliations
-
- 1Korea Cardiovascular Stent Research Institute, Jangsung, Korea. myungho@chollian.net
- 2Cardiovascular Convergence Research Center Nominated by Korea Ministry of Health and Welfare, Gwangju, Korea.
- 3Cardiovascular Research Center, Chonnam National University Hospital, Gwangju, Korea.
- 4Regeneromics Research Center, Chonnam National University, Gwangju, Gwangju, Korea.
- KMID: 2365290
- DOI: http://doi.org/10.4070/kcj.2016.0278
Abstract
- BACKGROUND AND OBJECTIVES
Artemisinin and dihydroartemisinin are drugs used to treat malaria. These drugs suppress inflammatory reactions. The aim of this study was to examine the anti-intima hyperplasia effect of a novel drug-eluting stent with artemisinin or dihydroartemisinin in a porcine coronary restenosis model.
MATERIALS AND METHODS
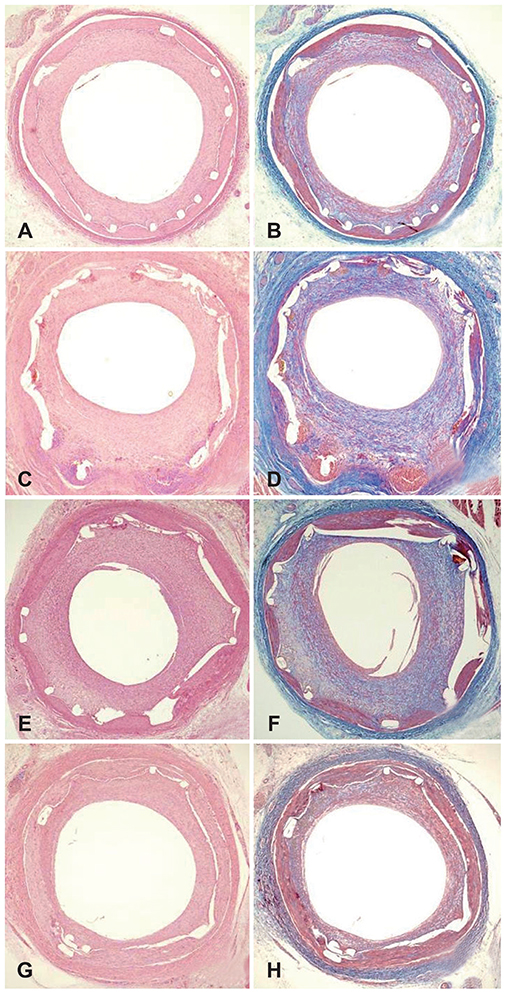
Pigs were randomized into four groups; in the first, the coronary arteries (20 pigs, a total of 40 coronary arteries, with 10 coronary arteries in each group) was implanted with bare metal stents (BMS, n=10); the second group was given polymer-coated stents (PCS, n=10); the third group was treated with artemisinin-eluting stents (AES, n=10); and the fourth group was given dihydroartemisinin-eluting stents (DAES, n=10). Histopathologic analysis was performed 28 days after stenting.
RESULTS
The injury and fibrin scores among the four groups were not significantly different. However, the internal elastic lamina, lumen area, and neointima area were significantly different. Moreover, the percent area of stenosis (46.2±18.66% in BMS vs. 89.4±10.92% in PCS vs. 83.3±17.07% in AES vs. 36.7±11.20% in DAES, p<0.0001) and inflammation score (1.0 [range: 1.0-1.0] vs. 3.0 [range: 2.25-3.0] vs. 3.0 [range: 1.0-3.0] vs. 2.0 [range: 1.75-3.0] in BMS, PCS, AES, and DAES, respectively; p<0.001) were markedly decreased in the DAES group compared to the PCS group.
CONCLUSION
DES, which uses a natural substance, dihydroartemisinin, showed a neointima and inflammatory suppressive effect in a porcine coronary restenosis model.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Can Artemisinin Be a Game Changer Even as an Antiarrhythmic Drug?
Jongmin Hwang
Korean Circ J. 2023;53(4):251-253. doi: 10.4070/kcj.2023.0031.
Reference
-
1. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994; 331:496–501.2. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994; 331:489–495.3. Cutlip DE, Chauhan MS, Baim DS, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002; 40:2082–2089.4. Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007; 370:937–948.5. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356:998–1008.6. Bae IH, Lim KS, Park JK, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Ind Eng Chem. 2015; 21:1295–1300.7. Lim KS, Bae IH, Kim JH, et al. Mechanical and histopathological comparison between commercialized and newly designed coronary bare metal stents in a porcine coronary restenosis model. Chonnam Med J. 2013; 49:7–13.8. Ma X, Oyamada S, Gao F, et al. Paclitaxel/sirolimus combination coated drug-eluting stent: in vitro and in vivo drug release studies. J Pharm Biomed Anal. 2011; 54:807–811.9. Lim KS, Park JK, Jeong MH, et al. Biodegradable polymer-based sirolimus coating stent in a porcine coronary restenosis model. Clin Exp Thromb Hemost. 2014; 1:59–65.10. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992; 19:267–274.11. Che HL, Bae IH, Lim KS, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012; 33:8548–8556.12. Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008; 1:143–153.13. Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001; 104:1188–1193.14. Lim KS, Park JK, Jeong MH, et al. Comparison of sirolimus loaded PLGA-PEG co-polymer coronary stent and bare metal stent in a porcine coronary restenosis model. Macromol Res. 2014; 22:639–646.15. Kang SN, Kim SE, Choi J, et al. Comparison of phytoncide with sirolimus as a novel drug candidate for drug-eluting stent. Biomaterials. 2015; 44:1–10.16. Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of instent restenosis: classification and implications for long-term outcome. Circulation. 1999; 100:1872–1878.17. Sollott SJ, Cheng L, Pauly RR, et al. Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J Clin Invest. 1995; 95:1869–1876.18. Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009; 360:1946–1959.19. De Luca G, Dirksen MT, Kelbaek H, et al. Paclitaxel-eluting versus bare metal stents in primary PCI: a pooled patient-level meta-analysis of randomized trials. J Thromb Thrombolysis. 2015; 39:101–112.20. Cao Q, Jiang Y, Shi J, et al. Artemisinin inhibits tumour necrosis factor-alpha-induced vascular smooth muscle cell proliferation in vitro and attenuates balloon injury-induced neointima formation in rats. Clin Exp Pharmacol Physiol. 2015; 42:502–509.21. Cao Q, Jiang Y, Shi J, et al. Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-alpha in vascular smooth muscle cells through nuclear factor kappa B pathway. J Surg Res. 2015; 194:667–678.22. Lee KP, Park ES, Kim DE, Park IS, Kim JT, Hong H. Artemisinin attenuates platelet-derived growth factor BB-induced migration of vascular smooth muscle cells. Nutr Res Pract. 2014; 8:521–525.23. Yang D, Yuan W, Lv C, et al. Dihydroartemisinin supresses inflammation and fibrosis in bleomycine-induced pulmonary fibrosis in rats. Int J Clin Exp Pathol. 2015; 8:1270–1281.24. Yu WY, Kan WJ, Yu PX, Li MM, Song JS, Zhao F. Anti-inflammatory effect and mechanism of artemisinin and dihydroartemisinin. Zhongguo Zhong Yao Za Zhi. 2012; 37:2618–2621.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The Effects of the Heparin-Coated Maximum Arterial Re-Creation (MAC) Stent on Porcine Coronary Stent Restenosis
- Anti-inflammatory Effect of Abciximab-Coated Stent in a Porcine Coronary Restenosis Model
- Effect of a Dual Drug-Coated Stent With Abciximab and Alpha-Lipoic Acid in a Porcine Coronary Restenosis Model