Pediatr Gastroenterol Hepatol Nutr.
2016 Dec;19(4):221-228. 10.5223/pghn.2016.19.4.221.
Microbiome-Linked Crosstalk in the Gastrointestinal Exposome towards Host Health and Disease
- Affiliations
-
- 1Laboratory of Mucosal Exposome and Biomodulation, Department of Biomedical Sciences, Pusan National University School of Medicine, Yangsan, Korea. moon@pnu.edu
- 2Research Institute for Basic Sciences and Medical Research Institute, Pusan National University, Busan, Korea.
- 3Immunoregulatory Therapeutics Group in Brain Busan 21 Project, Busan, Korea.
- KMID: 2364762
- DOI: http://doi.org/10.5223/pghn.2016.19.4.221
Abstract
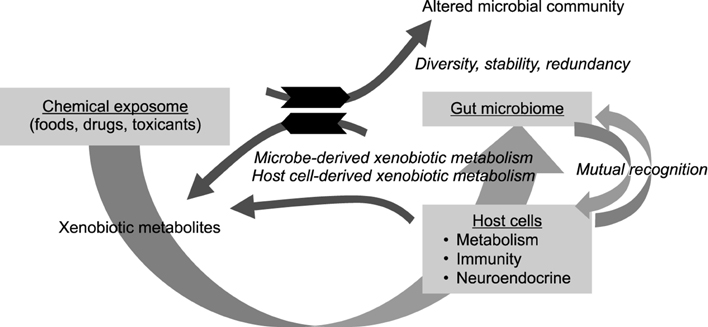
- The gastrointestinal exposome represents the integration of all xenobiotic components and host-derived endogenous components affecting the host health, disease progression and ultimately clinical outcomes during the lifespan. The human gut microbiome as a dynamic exposome of commensalism continuously interacts with other exogenous exposome as well as host sentineling components including the immune and neuroendocrine circuit. The composition and diversity of the microbiome are established on the basis of the luminal environment (physical, chemical and biological exposome) and host surveillance at each part of the gastrointestinal lining. Whereas the chemical exposome derived from nutrients and other xenobiotics can influence the dynamics of microbiome community (the stability, diversity, or resilience), the microbiomes reciprocally alter the bioavailability and activities of the chemical exposome in the mucosa. In particular, xenobiotic metabolites by the gut microbial enzymes can be either beneficial or detrimental to the host health although xenobiotics can alter the composition and diversity of the gut microbiome. The integration of the mucosal crosstalk in the exposome determines the fate of microbiome community and host response to the etiologic factors of disease. Therefore, the network between microbiome and other mucosal exposome would provide new insights into the clinical intervention against the mucosal or systemic disorders via regulation of the gut-associated immunological, metabolic, or neuroendocrine system.
Keyword
MeSH Terms
Figure
Reference
-
1. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011; 473:174–180.
Article2. Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, et al. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput Biol. 2013; 9:e1002863.
Article3. Raymond F, Ouameur AA, Déraspe M, Iqbal N, Gingras H, Dridi B, et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016; 10:707–720.
Article4. Kang C, Zhang Y, Zhu X, Liu K, Wang X, Chen M, et al. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab. 2016; 101:4681–4689.
Article5. Gibson MK, Pesesky MW, Dantas G. The yin and yang of bacterial resilience in the human gut microbiota. J Mol Biol. 2014; 426:3866–3876.
Article6. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012; 489:220–230.
Article7. Fiocchi C. Towards a ‘cure’ for IBD. Dig Dis. 2012; 30:428–433.
Article8. Fiocchi C. Integrating omics: the future of IBD? Dig Dis. 2014; 32:Suppl 1. 96–102.
Article9. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009; 137:1716–1724. e1-2.
Article10. Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011; 5:220–230.
Article11. Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016; 14:20–32.
Article12. Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol. 2011; 17:557–566.
Article13. Scharl M, Rogler G. Microbial sensing by the intestinal epithelium in the pathogenesis of inflammatory bowel disease. Int J Inflam. 2010; 2010:671258.
Article14. Scanlan PD, Shanahan F, O'Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn's disease. J Clin Microbiol. 2006; 44:3980–3988.
Article15. Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:675–683.
Article16. Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002; 277:20431–20437.
Article17. Awad WA, Ghareeb K, Bohm J, Zentek J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010; 27:510–520.
Article18. Yu H, Zhou T, Gong J, Young C, Su X, Li XZ, et al. Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCRDGGE guided microbial selection. BMC Microbiol. 2010; 10:182.
Article19. Tenk I, Fodor E, Szathmáry C. The effect of pure Fusarium toxins (T-2, F-2, DAS) on the microflora of the gut and on plasma glucocorticoid levels in rat and swine. Zentralbl Bakteriol Mikrobiol Hyg A. 1982; 252:384–393.
Article20. Waché YJ, Valat C, Postollec G, Bougeard S, Burel C, Oswald IP, et al. Impact of deoxynivalenol on the intestinal microflora of pigs. Int J Mol Sci. 2009; 10:1–17.
Article21. Bezirtzoglou EE. Intestinal cytochromes P450 regulating the intestinal microbiota and its probiotic profile. Microb Ecol Health Dis. 2012; 23:10. DOI: 10.3402/mehd.v23io.18370.
Article22. Lei L, Waterman MR, Fulco AJ, Kelly SL, Lamb DC. Availability of specific reductases controls the temporal activity of the cytochrome P450 complement of Streptomyces coelicolor A3(2). Proc Natl Acad Sci U S A. 2004; 101:494–499.
Article23. Sperry JF, Wilkins TD. Presence of cytochrome c in Desulfomonas pigra. J Bacteriol. 1977; 129:554–555.
Article24. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013; 54:2325–2340.
Article25. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008; 27:104–119.
Article26. Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011; 45:Suppl. S120–S127.27. Schilderink R, Verseijden C, de Jonge WJ. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol. 2013; 4:226.
Article28. Schilderink R, Verseijden C, Seppen J, Muncan V, van den Brink GR, Lambers TT, et al. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am J Physiol Gastrointest Liver Physiol. 2016; 310:G1138–G1146.
Article29. Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004; 317:463–471.
Article30. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014; 40:833–842.
Article31. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011; 141:599–609. 609.e1–609.e3.
Article32. Duca FA, Lam TK. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab. 2014; 16:Suppl 1. 68–76.
Article33. Paul HA, Bomhof MR, Vogel HJ, Reimer RA. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci Rep. 2016; 6:20683.
Article34. Reid DT, Eller LK, Nettleton JE, Reimer RA. Postnatal prebiotic fibre intake mitigates some detrimental metabolic outcomes of early overnutrition in rats. Eur J Nutr. 2016; 55:2399–2409.
Article35. Yang J, Summanen PH, Henning SM, Hsu M, Lam H, Huang J, et al. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front Physiol. 2015; 6:216.
Article36. Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015; 148:1107–1119.
Article37. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013; 39:372–385.
Article38. Ikuta T, Kurosumi M, Yatsuoka T, Nishimura Y. Tissue distribution of aryl hydrocarbon receptor in the intestine: Implication of putative roles in tumor suppression. Exp Cell Res. 2016; 343:126–134.
Article39. Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015; 67:259–279.
Article40. Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014; 14:801–814.
Article41. Hartiala J, Bennett BJ, Tang WH, Wang Z, Stewart AF, Roberts R, et al. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler Thromb Vasc Biol. 2014; 34:1307–1313.
Article42. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011; 472:57–63.
Article43. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013; 19:576–585.
Article44. Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011; 85:863–871.
Article45. Saracut C, Molnar C, Russu C, Todoran N, Vlase L, Turdean S, et al. Secondary bile acids effects in colon pathology. Experimental mice study. Acta Cir Bras. 2015; 30:624–631.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mental Disorders Linked to Crosstalk between The Gut Microbiome and The Brain
- Iron Reshapes the Gut Microbiome and Host Metabolism
- Importance of nutritional therapy in the management of intestinal diseases: beyond energy and nutrient supply
- Human microbiome studies in Korea
- The exposome and the future of epidemiology: a vision and prospect