Korean J Ophthalmol.
2015 Oct;29(5):315-324. 10.3341/kjo.2015.29.5.315.
Clinical Outcomes of Eyes with Submacular Hemorrhage Secondary to Age-related Macular Degeneration Treated with Anti-vascular Endothelial Growth Factor
- Affiliations
-
- 1Department of Ophthalmology, Kim's Eye Hospital, Konyang University College of Medicine, Seoul, Korea. kimoph@gmail.com
- 2Department of Ophthalmology, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2363805
- DOI: http://doi.org/10.3341/kjo.2015.29.5.315
Abstract
- PURPOSE
To evaluate the long-term outcomes of intravitreal anti-vascular endothelial growth factor (VEGF) monotherapy for patients diagnosed with submacular hemorrhage secondary to exudative age-related macular degeneration.
METHODS
This retrospective, observational study included 49 patients (49 eyes) who initially presented with submacular hemorrhage associated with exudative age-related macular degeneration and who were followed-up for at least 24 months. Only eyes that were treated with intravitreal anti-VEGF monotherapy were included in the study. Best-corrected visual acuity (BCVA) measurements obtained at diagnosis, six months, and the final visit were compared. The associations of BCVA at the final visit with baseline BCVA, BCVA at six months, symptom duration, hemorrhage extent, and central foveal thickness were also analyzed.
RESULTS
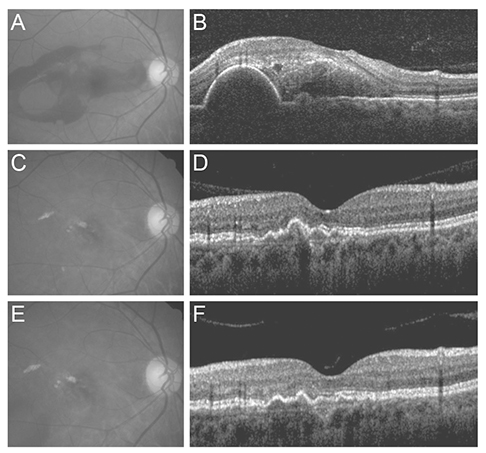
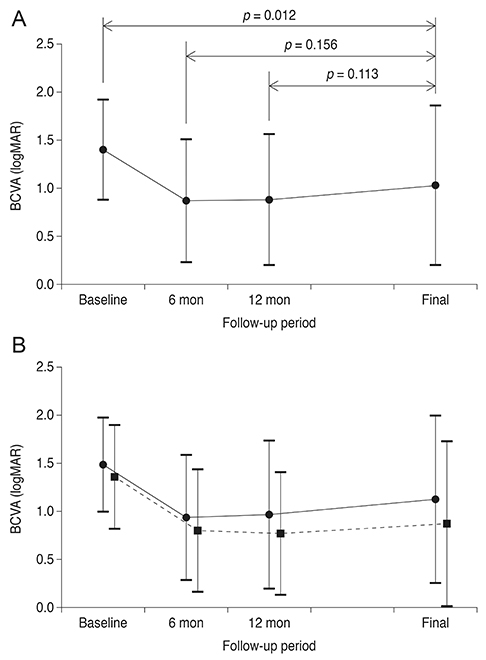
Over the course of follow-up (mean, 32.1 +/- 8.5 months), an average of 5.1 +/- 2.2 anti-VEGF injections were administered. Recurrent hemorrhage was noted in 13 eyes (26.5%). The mean logarithm of the minimal angle of resolution BCVA at diagnosis, six months, and the final visit were 1.40 +/- 0.52, 0.87 +/- 0.64, and 1.03 +/- 0.83, respectively. Both baseline BCVA (p = 0.012) and BCVA at six months (p < 0.001) were significantly associated with BCVA at the final visit.
CONCLUSIONS
Improved visual acuity was maintained for more than two years with intravitreal anti-VEGF monotherapy. BCVA at six months is a useful clinical index to predict long-term visual prognosis.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Angiogenesis Inhibitors/administration & dosage
Bevacizumab/*administration & dosage
Female
Fluorescein Angiography
Follow-Up Studies
Fundus Oculi
Humans
Intravitreal Injections
Male
Middle Aged
Ranibizumab/*administration & dosage
Retina/*diagnostic imaging
Retinal Hemorrhage/diagnosis/*drug therapy/etiology
Retrospective Studies
Time Factors
Tomography, Optical Coherence
Treatment Outcome
Vascular Endothelial Growth Factor A/*antagonists & inhibitors
Visual Acuity
Wet Macular Degeneration/complications/diagnosis/*drug therapy
Angiogenesis Inhibitors
Bevacizumab
Ranibizumab
Vascular Endothelial Growth Factor A
Figure
Cited by 2 articles
-
Intravitreal Aflibercept Monotherapy for Treating Submacular Hemorrhage Secondary to Neovascular Age-related Macular Degeneration
Sue Hey Chae, Soh Eun Ahn, Hee Seong Yoon
J Korean Ophthalmol Soc. 2018;59(5):437-443. doi: 10.3341/jkos.2018.59.5.437.Development of Submacular Hemorrhage in Neovascular Age-related Macular Degeneration: Influence on Visual Prognosis in a Clinical Setting
Young Suk Chang, Jae Hui Kim, Jong Woo Kim, Chul Gu Kim, Dong Won Lee
Korean J Ophthalmol. 2018;32(5):361-368. doi: 10.3341/kjo.2017.0095.
Reference
-
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.2. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–372.e5.3. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–583.4. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398.5. Kwon YH, Lee DK, Kim HE, Kwon OW. Predictive findings of visual outcome in spectral domain optical coherence tomography after ranibizumab treatment in age-related macular degeneration. Korean J Ophthalmol. 2014; 28:386–392.6. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.7. Cho HJ, Koh KM, Kim HS, et al. Anti-vascular endothelial growth factor monotherapy in the treatment of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013; 156:524–531.e1.8. Iacono P, Parodi MB, Introini U, et al. Intravitreal ranibizumab for choroidal neovascularization with large submacular hemorrhage in age-related macular degeneration. Retina. 2014; 34:281–287.9. Shienbaum G, Garcia Filho CA, Flynn HW Jr, et al. Management of submacular hemorrhage secondary to neovascular age-related macular degeneration with anti-vascular endothelial growth factor monotherapy. Am J Ophthalmol. 2013; 155:1009–1013.10. Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol. 2007; 144:886–892.11. Kim JH, Chang YS, Kim JW, et al. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology. 2014; 121:926–935.12. McKibbin M, Papastefanou V, Matthews B, et al. Ranibizumab monotherapy for sub-foveal haemorrhage secondary to choroidal neovascularisation in age-related macular degeneration. Eye (Lond). 2010; 24:994–998.13. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996; 16:183–189.14. Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004; 111:1993–2006.15. Scupola A, Coscas G, Soubrane G, Balestrazzi E. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999; 213:97–102.16. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990; 109:33–37.17. Hwang JU, Yang SJ, Yoon YH, et al. Recurrent submacular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2012; 32:652–657.18. Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004; 30:287–290.19. Kang SW, Chung SE, Shin WJ, Lee JH. Polypoidal choroidal vasculopathy and late geographic hyperfluorescence on indocyanine green angiography. Br J Ophthalmol. 2009; 93:759–764.20. Kim JH, Kang SW, Kim TH, et al. Structure of polypoidal choroidal vasculopathy studied by colocalization between tomographic and angiographic lesions. Am J Ophthalmol. 2013; 156:974–980.e2.21. Sato T, Kishi S, Watanabe G, et al. Tomographic features of branching vascular networks in polypoidal choroidal vasculopathy. Retina. 2007; 27:589–594.22. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.23. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990; 10:1–8.24. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004; 49:25–37.25. Coscas G, Yamashiro K, Coscas F, et al. Comparison of exudative age-related macular degeneration subtypes in Japanese and French Patients: multicenter diagnosis with multimodal imaging. Am J Ophthalmol. 2014; 158:309–318.e2.26. Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121:1392–1396.27. Byeon SH, Lee SC, Oh HS, et al. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008; 52:57–62.28. Koh AH. Expert PCV Panel. Chen LJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013; 33:686–716.29. Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013; 156:644–651.30. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2008; 22:92–99.31. Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012; 32:1453–1464.32. Yoneyama S, Sakurada Y, Mabuchi F, et al. Genetic and clinical factors associated with reticular pseudodrusen in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2014; 252:1435–1441.33. Fujimura S, Ueta T, Takahashi H, et al. Characteristics of fundus autofluorescence and drusen in the fellow eyes of Japanese patients with exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2013; 251:1–9.34. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011; 31:1904–1911.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Acute Submacular Hemorrhage with Intravitreal Injection of Tenecteplase, Anti-vascular Endothelial Growth Factor and Gas
- Development of Submacular Hemorrhage in Neovascular Age-related Macular Degeneration: Influence on Visual Prognosis in a Clinical Setting
- Intravitreal Aflibercept Monotherapy for Treating Submacular Hemorrhage Secondary to Neovascular Age-related Macular Degeneration
- Treatment of Exudative Age-Related Macular Degeneration
- Combined Anti-VEGF and C3F8 Injection for Large Submacular Hemorrhage Secondary to Age-Related Macular Degeneration