J Periodontal Implant Sci.
2016 Dec;46(6):382-395. 10.5051/jpis.2016.46.6.382.
Effectiveness of alendronate as an adjunct to scaling and root planing in the treatment of periodontitis: a meta-analysis of randomized controlled clinical trials
- Affiliations
-
- 1Chongqing Key Laboratory for Oral Diseases and Biomedical Sciences, Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing Medical University College of Stomatology, Chongqing, China. soongjl@163.com
- KMID: 2362904
- DOI: http://doi.org/10.5051/jpis.2016.46.6.382
Abstract
- PURPOSE
Alendronate has been proposed as a local and systemic drug treatment used as an adjunct to scaling and root planing (SRP) for the treatment of periodontitis. However, its effectiveness has yet to be conclusively established. The purpose of the present meta-analysis was to assess the effectiveness of SRP with alendronate on periodontitis compared to SRP alone.
METHODS
Five electronic databases were used by 2 independent reviewers to identify relevant articles from the earliest records up to September 2016. Randomized controlled trials (RCTs) comparing SRP with alendronate to SRP with placebo in the treatment of periodontitis were included. The outcome measures were changes in bone defect fill, probing depth (PD), and clinical attachment level (CAL) from baseline to 6 months. A fixed-effect or random-effect model was used to pool the extracted data, as appropriate. Mean differences (MDs) with 95% confidence intervals (CIs) were calculated. Heterogeneity was assessed using the Cochrane χ² and I2 tests.
RESULTS
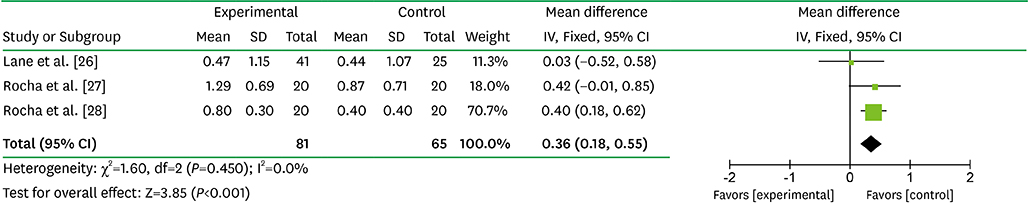
After the selection process, 8 articles were included in the meta-analysis. Compared with SRP alone, the adjunctive mean benefits of locally delivered alendronate were 38.25% for bone defect fill increase (95% CI=33.05-43.45; P<0.001; I²=94.0%), 2.29 mm for PD reduction (95% CI=2.07-2.52 mm; P<0.001; I²=0.0%) and 1.92 mm for CAL gain (95% CI=1.55-2.30 mm; P<0.001; I²=66.0%). In addition, systemically administered alendronate with SRP significantly reduced PD by 0.36 mm (95% CI=0.18-0.55 mm; P<0.001; I²=0.0%) and increased CAL by 0.39 mm (95% CI=0.11-0.68 mm; P=0.006; I²=6.0%).
CONCLUSIONS
The collective evidence regarding the adjunctive use of alendronate locally and systemically with SRP indicates that the combined treatment can improve the efficacy of non-surgical periodontal therapy on increasing CAL and bone defect fill and reducing PD. However, precautions must be exercised in interpreting these results, and multicenter studies evaluating this specific application should be carried out.
Keyword
MeSH Terms
Figure
Reference
-
1. Periodontal diseases: pathogenesis and microbial factors. J Am Dent Assoc. 1998; 129:Suppl. 58S–62S.2. Armitage GC, Robertson PB. The biology, prevention, diagnosis and treatment of periodontal diseases: scientific advances in the United States. J Am Dent Assoc. 2009; 140:Suppl 1. 36S–43S.3. Lindhe J, Nyman S. The effect of plaque control and surgical pocket elimination on the establishment and maintenance of periodontal health. A longitudinal study of periodontal therapy in cases of advanced disease. J Clin Periodontol. 1975; 2:67–79.
Article4. Hill RW, Ramfjord SP, Morrison EC, Appleberry EA, Caffesse RG, Kerry GJ, et al. Four types of periodontal treatment compared over two years. J Periodontol. 1981; 52:655–662.
Article5. Ramfjord SP, Caffesse RG, Morrison EC, Hill RW, Kerry GJ, Appleberry EA, et al. Four modalities of periodontal treatment compared over five years. J Periodontal Res. 1987; 22:222–223.
Article6. Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005; 32:Suppl 6. 108–129.
Article7. Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996; 1:821–878.
Article8. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008; 79:1585–1591.
Article9. Ciancio SG. Systemic medications: clinical significance in periodontics. J Clin Periodontol. 2002; 29:Suppl 2. 17–21.
Article10. Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003; 5:65–74.
Article11. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000; 88:2961–2978.
Article12. Binderman I, Adut M, Yaffe A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. J Periodontol. 2000; 71:1236–1240.
Article13. Tenenbaum HC, Shelemay A, Girard B, Zohar R, Fritz PC. Bisphosphonates and periodontics: potential applications for regulation of bone mass in the periodontium and other therapeutic/diagnostic uses. J Periodontol. 2002; 73:813–822.
Article14. Weinreb M, Quartuccio H, Seedor JG, Aufdemorte TB, Brunsvold M, Chaves E, et al. Histomorphometrical analysis of the effects of the bisphosphonate alendronate on bone loss caused by experimental periodontitis in monkeys. J Periodontal Res. 1994; 29:35–40.
Article15. Hu JH, Ding M, Søballe K, Bechtold JE, Danielsen CC, Day JS, et al. Effects of short-term alendronate treatment on the three-dimensional microstructural, physical, and mechanical properties of dog trabecular bone. Bone. 2002; 31:591–597.
Article16. Veena HR, Prasad D. Evaluation of an aminobisphosphonate (alendronate) in the management of periodontal osseous defects. J Indian Soc Periodontol. 2010; 14:40–45.
Article17. Kruszewska H, Zareba T, Tyski S. Search of antimicrobial activity of selected non-antibiotic drugs. Acta Pol Pharm. 2002; 59:436–439.18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62:e1–34.
Article19. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009; 62:1006–1012.
Article20. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Oxford: The Cochrane Collaboration;2011.21. Pradeep AR, Kumari M, Rao NS, Naik SB. 1% alendronate gel as local drug delivery in the treatment of Class II furcation defects: a randomized controlled clinical trial. J Periodontol. 2013; 84:307–315.
Article22. Sharma A, Pradeep AR. Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: a randomized, controlled clinical trial. J Periodontol. 2012; 83:11–18.
Article23. Sharma A, Pradeep AR. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: a randomized controlled clinical trial. J Periodontol. 2012; 83:19–26.
Article24. Pradeep AR, Sharma A, Rao NS, Bajaj P, Naik SB, Kumari M. Local drug delivery of alendronate gel for the treatment of patients with chronic periodontitis with diabetes mellitus: a double-masked controlled clinical trial. J Periodontol. 2012; 83:1322–1328.
Article25. Pradeep AR, Kanoriya D, Singhal S, Garg V, Manohar B, Chatterjee A. Comparative evaluation of subgingivally delivered 1% alendronate versus 1.2% atorvastatin gel in treatment of chronic periodontitis: a randomized placebo-controlled clinical trial. J Investig Clin Dent. Forthcoming. 2016.
Article26. Lane N, Armitage GC, Loomer P, Hsieh S, Majumdar S, Wang HY, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol. 2005; 76:1113–1122.
Article27. Rocha M, Nava LE, Vázquez de la Torre C, Sánchez-Márin F, Garay-Sevilla ME, Malacara JM. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo-controlled trial. J Periodontol. 2001; 72:204–209.
Article28. Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol. 2004; 75:1579–1585.
Article29. Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005; 353:99–102.
Article30. De Almeida J, Ervolino E, Bonfietti LH, Novaes VC, Theodoro LH, Fernandes LA, et al. Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. J Periodontol. 2015; 86:1166–1175.
Article31. Menezes AM, Rocha FA, Chaves HV, Carvalho CB, Ribeiro RA, Brito GA. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005; 76:1901–1909.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effectiveness of Subgingival Scaling and Root Planing via Closed Approach in Calculus Removal
- Clinical and microbiologic effects of the subantimicrobial dose of doxycycline on the chronic periodontitis
- Effects of Periodontal Treatment on Glycated Hemoglobin A Levels in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials
- The Clinical and Microbiological Study of the Effect of Minocycline Strip Locally Administrated on Adult Periodontitis
- Effects of 2% minocycline gel as an adjunct to scaling and root planing on the treatment of adult periodontitis