Diabetes Metab J.
2016 Dec;40(6):494-508. 10.4093/dmj.2016.40.6.494.
Investigating Susceptibility to Diabetes Using Features of the Adipose Tissue in Response to In Utero Polycyclic Aromatic Hydrocarbons Exposure
- Affiliations
-
- 1Department of Chemistry, Georgia Southern University, Statesboro, GA, USA. wgato@georgiasouthern.edu
- 2Department of Pathology, University of Georgia College of Veterinary Medicine, Athens, GA, USA.
- KMID: 2362222
- DOI: http://doi.org/10.4093/dmj.2016.40.6.494
Abstract
- BACKGROUND
In recent times, there has been an increase in the incidence of type 2 diabetes mellitus (T2DM) particularly in children. Adipocyte dysfunction provide a critical link between obesity and insulin resistance resulting in diabetes outcome. Further, environmental chemical exposure during early years of life might be a significant contributing factor to the increase in the incidence of T2DM. This study tests the idea that exposure to environmental contaminants (2-aminoanthracene [2AA]) in utero will show effects in the adipose tissue (AT) that signify T2DM vulnerability. 2AA is a polycyclic aromatic hydrocarbon found in a variety of products.
METHODS
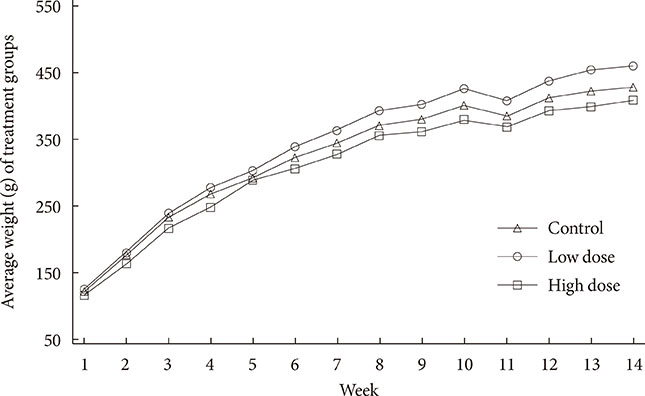
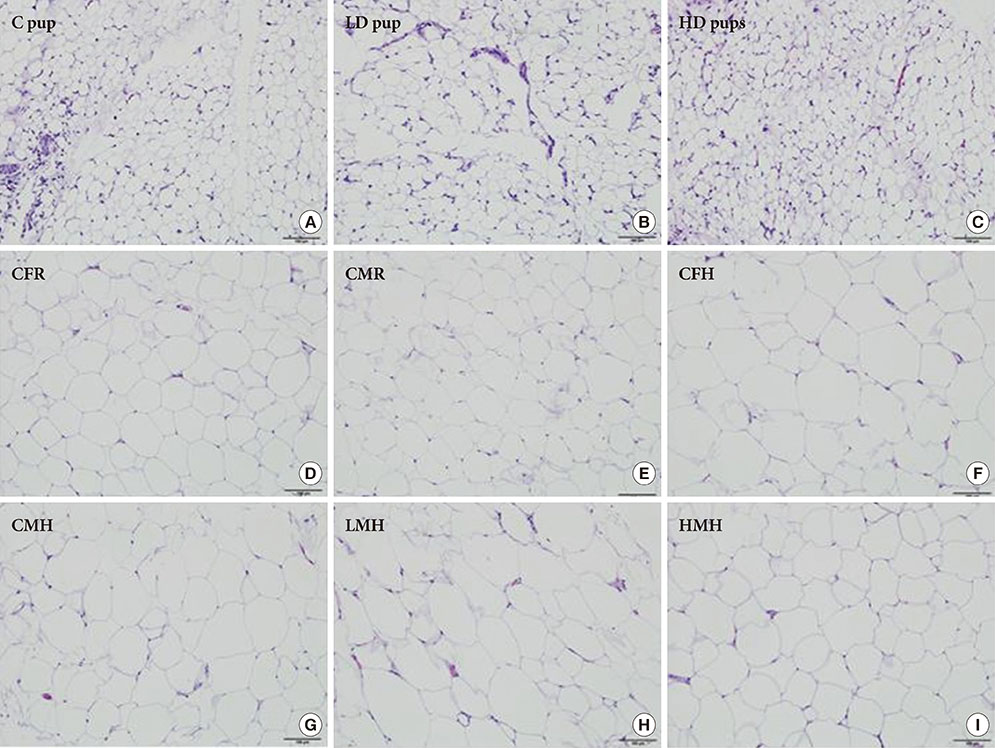
To accomplish the study objective, pregnant dams were fed various amounts of 2AA adulterated diets from gestation through postnatal period. The neonates and older offspring were analyzed for diabetic-like genes in the ATs and analysis of serum glucose. Furthermore, weight monitoring, histopathology and immunohistochemical (IHC) staining for CD68 in AT, adipocyte size determination and adiponectin amounts in serum were undertaken.
RESULTS
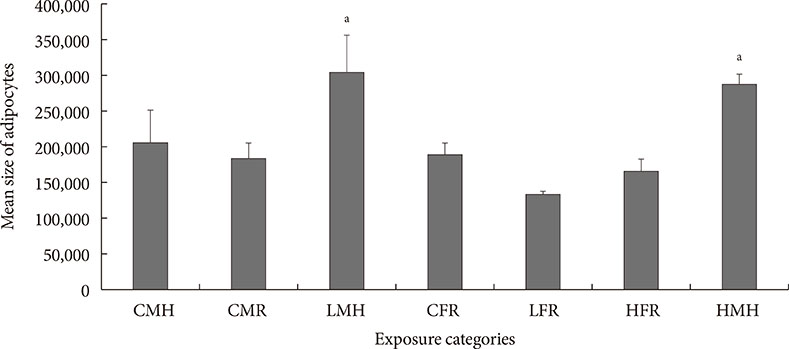
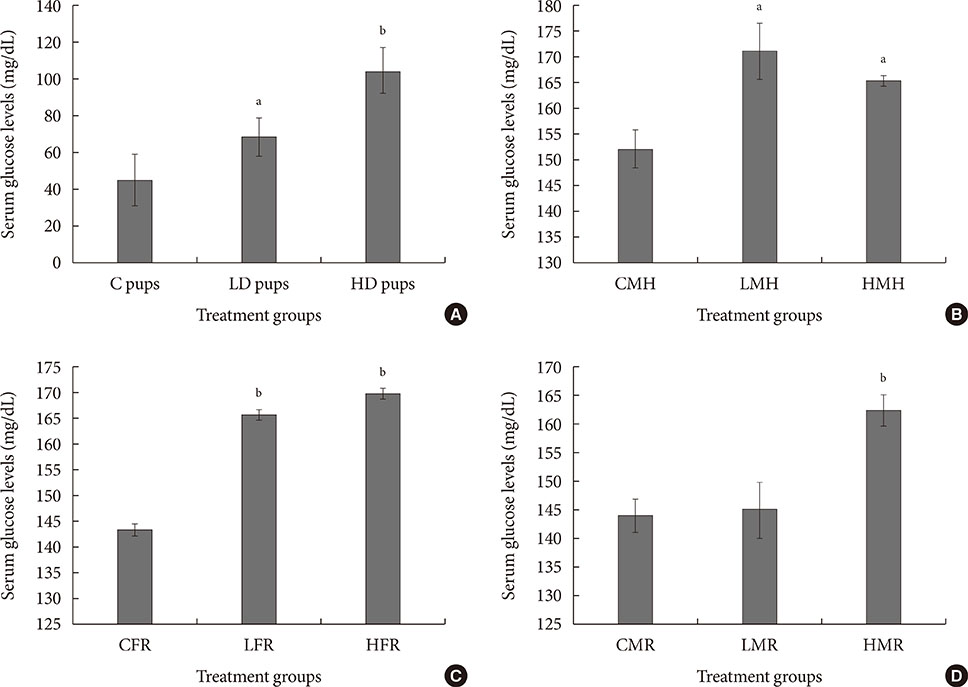
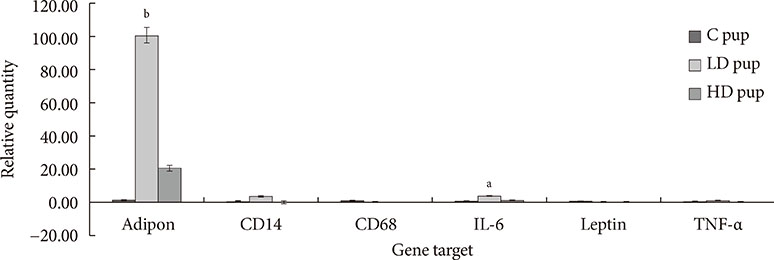
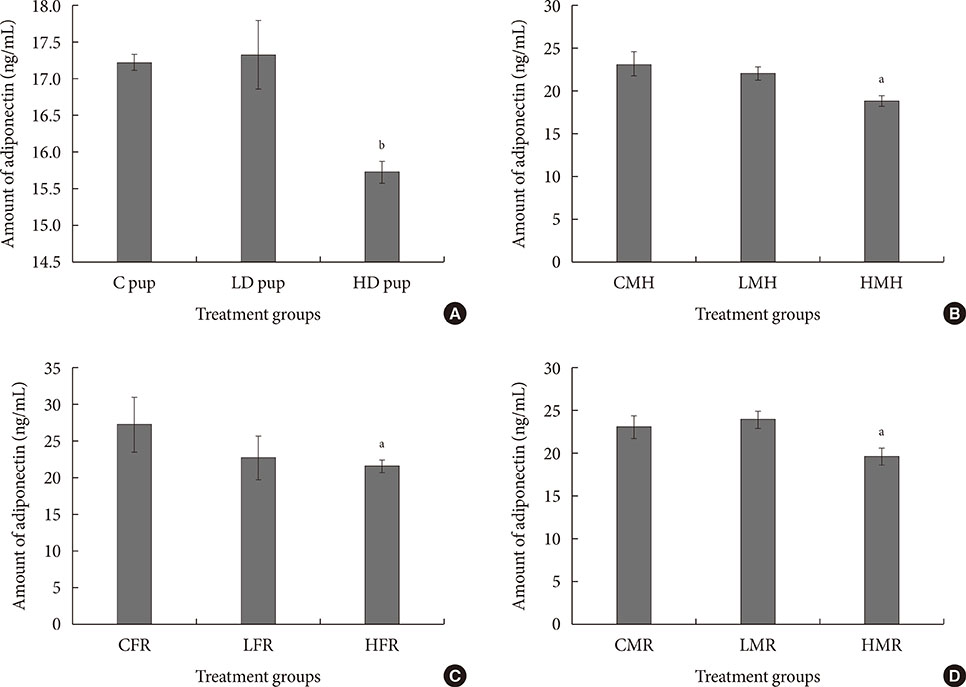
Up-regulation of adiponectin and interleukin-6 genes were noted in the pups and older rats. Combination of intrauterine 2AA toxicity with moderate high fat diet exhibited gene expression patterns similar to those of the neonates. Elevated serum glucose levels were noted in treated groups. IHC of the AT indicated no significant malformations; however, CD68+ cells were greater in the animals treated to 2AA. Similarly, mean sizes of the adipocytes were larger in treated and combined 2AA and moderate high fat animals. Adiponectin was reduced in 2AA groups.
CONCLUSION
From the preceding, it appears intrauterine 2AA disturbance, when combined with excess fat accumulation will lead to greater risk for the diabetic condition.
Keyword
MeSH Terms
-
Adipocytes
Adiponectin
Adipose Tissue*
Animals
Blood Glucose
Child
Diabetes Mellitus, Type 2
Diet
Diet, High-Fat
Gene Expression
Humans
Incidence
Infant, Newborn
Insulin Resistance
Interleukin-6
Obesity
Polycyclic Hydrocarbons, Aromatic*
Pregnancy
Rats
Up-Regulation
Adiponectin
Interleukin-6
Polycyclic Hydrocarbons, Aromatic
Figure
Reference
-
1. Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. The continuing increase of diabetes in the US. Diabetes Care. 2001; 24:412.2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:1047–1053.3. Hu FB. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids. 2003; 38:103–108.4. Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003; 289:1785–1791.5. Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005; 54:2305–2313.6. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115:1111–1119.7. Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, Peet A, Tillmann V, Poho P, Mattila I, Lahdesmaki H, Franzosa EA, Vaarala O, de Goffau M, Harmsen H, Ilonen J, Virtanen SM, Clish CB, Oresic M, Huttenhower C, Knip M. DIABIMMUNE Study Group. Xavier RJ. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015; 17:260–273.8. Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Cien Saude Colet. 2008; 13:269–281.9. Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012; 120:779–789.10. Thompson RF, Einstein FH. Epigenetic basis for fetal origins of age-related disease. J Womens Health (Larchmt). 2010; 19:581–587.11. Landrigan PJ, Carlson JE, Bearer CF, Cranmer JS, Bullard RD, Etzel RA, Groopman J, McLachlan JA, Perera FP, Reigart JR, Robison L, Schell L, Suk WA. Children's health and the environment: a new agenda for prevention research. Environ Health Perspect. 1998; 106:Suppl 3. 787–794.12. Landrigan PJ, Garg A. Chronic effects of toxic environmental exposures on children's health. J Toxicol Clin Toxicol. 2002; 40:449–456.13. Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000; 108:Suppl 3. 451–455.14. Bott S, Shafagoj YA, Sawicki PT, Heise T. Impact of smoking on the metabolic action of subcutaneous regular insulin in type 2 diabetic patients. Horm Metab Res. 2005; 37:445–449.15. Boudreau MD, Taylor HW, Baker DG, Means JC. Dietary exposure to 2-aminoanthracene induces morphological and immunocytochemical changes in pancreatic tissues of Fisher-344 rats. Toxicol Sci. 2006; 93:50–61.16. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830.17. Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000; 11:212–217.18. Swaroop JJ, Rajarajeswari D, Naidu JN. Association of TNF-alpha with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012; 135:127–130.19. Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002; 51:3391–3399.20. Pedersen BK, Febbraio MA. Point: interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol (1985). 2007; 102:814–816.21. Haluzik M, Parizkova J, Haluzik MM. Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res. 2004; 53:123–129.22. Stefan N, Stumvoll M. Adiponectin: its role in metabolism and beyond. Horm Metab Res. 2002; 34:469–474.23. Fernandez-Real JM, Broch M, Richart C, Vendrell J, Lopez-Bermejo A, Ricart W. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab. 2003; 88:1780–1784.24. Fernandez-Real JM, Perez del Pulgar S, Luche E, Moreno-Navarrete JM, Waget A, Serino M, Sorianello E, Sanchez-Pla A, Pontaque FC, Vendrell J, Chacon MR, Ricart W, Burcelin R, Zorzano A. CD14 modulates inflammation-driven insulin resistance. Diabetes. 2011; 60:2179–2186.25. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007; 8:21–34.26. Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000; 59:359–371.27. US Department of Health and Human Services, Public Health Service, ATSDR Agency for Toxic Substances and Disease Registry. Toxicological profile for polycyclic aromatic hydrocarbons. cited 2016 Jul 20. Available from: http://www.atsdr.cdc.gov/ToxProfiles/tp69.pdf.28. Gato WE, Hales DB, Means JC. Hepatic gene expression analysis of 2-aminoanthracene exposed Fisher-344 rats reveal patterns indicative of liver carcinoma and type 2 diabetes. J Toxicol Sci. 2012; 37:1001–1016.29. StatsToDo. Computer program to calculate sample size requirement in the analysis of variance. cited 2016 Jul 20. Available from: http://www.statstodo.com/SSizAOV_Pgm.php#.30. Meek TH, Eisenmann JC, Garland T Jr. Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obes (Lond). 2010; 34:960–969.31. Wood WA, Kellogg ST. Chapter 40, Isolation of 1,4-b-D-glucan 4-glucanohydrolases of talaromyces emersonii. Methods in enzymology, vol. 160. London: London Academic Press;1988. p. 363–367.32. Wood WA, Kellogg ST. Chapter 9, Methods for measuring cellulase activities. Methods in enzymology, vol. 160. London: London Academic Press;1988. p. 87–111.33. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.34. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919.35. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007; 92:1023–1033.36. Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003; 26:372–379.37. Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010; 24:326–331.38. Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Kloting N, Gregoire C, Lolmede K, Bluher M, Clement K. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014; 99:E1466–E1470.39. Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes. 1996; 45:699–701.40. Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000; 105:1243–1252.41. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009; 52:1686–1688.42. Hernandez ML, Harris B, Lay JC, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, Peden DB. Comparative airway inflammatory response of normal volunteers to ozone and lipopolysaccharide challenge. Inhal Toxicol. 2010; 22:648–656.43. Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ Health. 2013; 12:73.44. De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012; 2012:713696.45. Upadhyaya S, Kadamkode V, Mahammed R, Doraiswami C, Banerjee G. Adiponectin and IL-6: mediators of inflammation in progression of healthy to type 2 diabetes in Indian population. Adipocyte. 2014; 3:39–45.46. Ndisang JF, Jadhav A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology. 2014; 155:215–229.47. Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm J. 2015; 23:250–256.48. Abdelgadir M, Karlsson AF, Berglund L, Berne C. Low serum adiponectin concentrations are associated with insulin sensitivity independent of obesity in Sudanese subjects with type 2 diabetes mellitus. Diabetol Metab Syndr. 2013; 5:15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Investigating Susceptibility to Diabetes Using Features of the Adipose Tissue in Response to In Utero Polycyclic Aromatic Hydrocarbons Exposure (Diabetes Metab J 2016;40:494-508)
- Response: Investigating Susceptibility to Diabetes Using Features of the Adipose Tissue in Response to In Utero Polycyclic Aromatic Hydrocarbons Exposure (Diabetes Metab J 2016;40:494-508)
- Association between Environmental Polycyclic Aromatic Hydrocarbons Exposure and Insulin Resistance: Using The Second Korean National Environmental Health Survey
- Preliminary Investigation into Urinary 1-Hydroxypyrene as a Biomarker for Polycyclic Aromatic Hydrocarbons exposure among Charcoal Workers in Ogun and Oyo States, Nigeria
- Relationship between PAHs Concentrations in Ambient Air and Deposited on Pine Needles