Cancer Res Treat.
2016 Oct;48(4):1274-1285. 10.4143/crt.2015.502.
Is There a Role for Adjuvant Therapy in R0 Resected Gallbladder Cancer?: A Propensity Score-Matched Analysis
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea. newatp@naver.com
- 2Division of Hematology/Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 3Division of Hematology/Oncology, Department of Internal Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea.
- 4Department of Radiology, Gachon University Gil Medical Center, Incheon, Korea.
- 5Division of Hematology/Oncology, Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 6Division of Hematology/Oncology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 7Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. oncopark@skku.edu
- KMID: 2356229
- DOI: http://doi.org/10.4143/crt.2015.502
Abstract
- PURPOSE
The purpose of this study is to assess the role of adjuvant therapy in stage I-III gallbladder cancer (GBC) patients who have undergone R0 resection.
MATERIALS AND METHODS
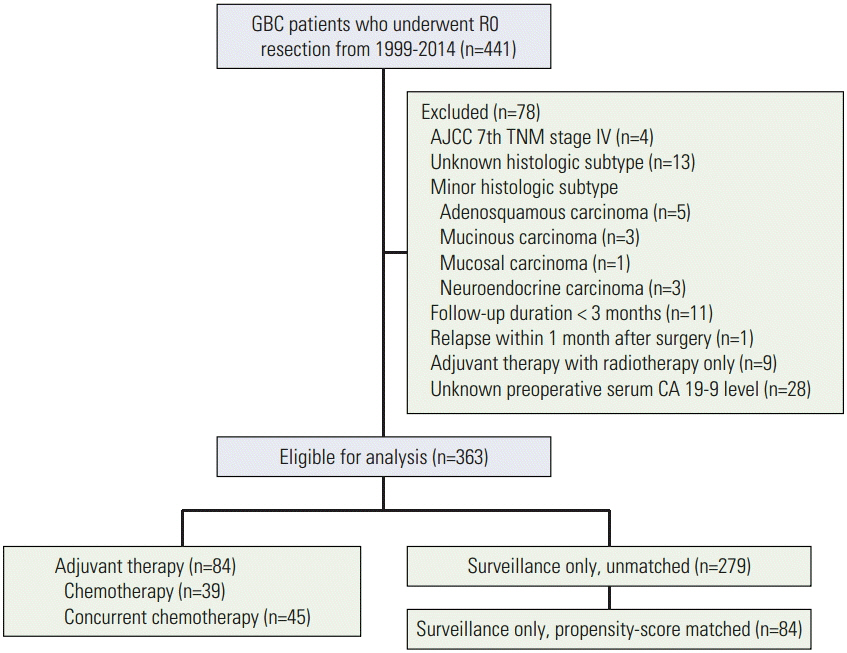
Clinical data were collected on 441 consecutive patients who underwent R0 resection for stage I-III GBC. Eligible patients were classified into adjuvant therapy and surveillance only groups. Propensity score matching (PSM) between the two groups was performed, adjusting clinical factors.
RESULTS
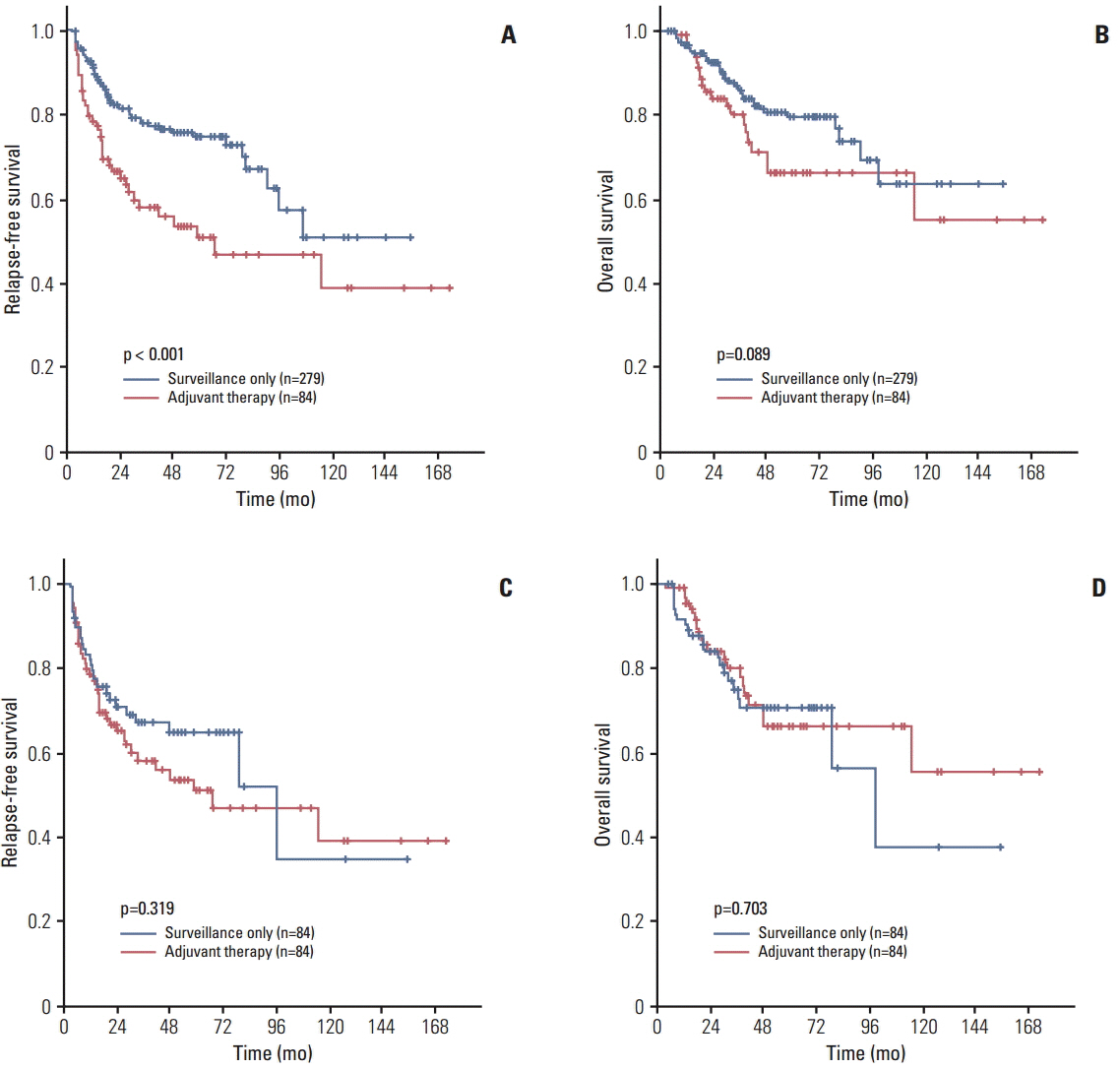
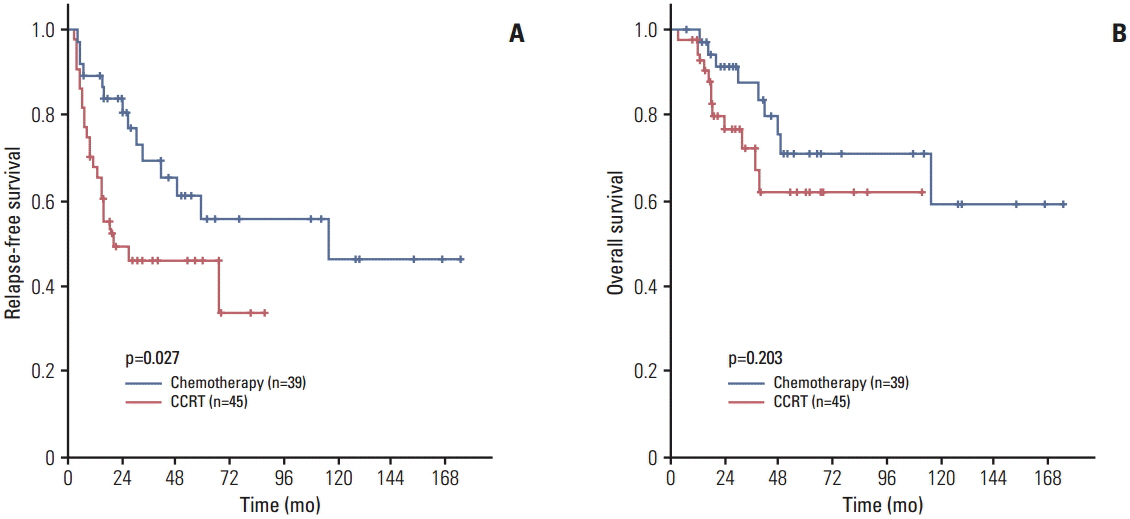
In total, 84 and 279 patients treated with adjuvant therapy and followed up with surveillance only, respectively, were included in the analysis. Before PSM, the 5-year relapse-free survival (RFS) rate was lower in the adjuvant therapy group than in the surveillance only group (50.8% vs. 74.8%, p < 0.001), although there was no statistically significant difference in the 5-year overall survival (OS) rate (66.2% vs. 79.5%, p=0.089). After the PSM, baseline characteristics became comparable and there were no differences in the 5-year RFS (50.8% vs. 64.8%, p=0.319) and OS (66.2% vs. 70.4%, p=0.703) rates between the two groups.
CONCLUSION
The results suggest that fluoropyrimidine-based adjuvant therapy is not indicated in stage I-III GBC patients who have undergone R0 resection.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
Article2. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–41.
Article3. Lim H, Seo DW, Park DH, Lee SS, Lee SK, Kim MH, et al. Prognostic factors in patients with gallbladder cancer after surgical resection: analysis of 279 operated patients. J Clin Gastroenterol. 2013; 47:443–8.4. Gold DG, Miller RC, Haddock MG, Gunderson LL, Quevedo F, Donohue JH, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2009; 75:150–5.
Article5. Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR Jr, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008; 26:2112–7.
Article6. Cereda S, Belli C, Reni M. Adjuvant treatment in biliary tract cancer: to treat or not to treat? World J Gastroenterol. 2012; 18:2591–6.
Article7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: hepatobiliary cancers [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2015. [cited 2015 Aug 16]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.8. Cho SY, Kim SH, Park SJ, Han SS, Kim YK, Lee KW, et al. Adjuvant chemoradiation therapy in gallbladder cancer. J Surg Oncol. 2010; 102:87–93.
Article9. Wang SJ, Lemieux A, Kalpathy-Cramer J, Ord CB, Walker GV, Fuller CD, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol. 2011; 29:4627–32.
Article10. Nakamura M, Nakashima H, Abe T, Ensako T, Yoshida K, Hino K. Gemcitabine-based adjuvant chemotherapy for patients with advanced gallbladder cancer. Anticancer Res. 2014; 34:3125–9.11. Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002; 95:1685–95.12. Park HS, Lim JY, Yoon DS, Park JS, Lee DK, Lee SJ, et al. Outcome of adjuvant therapy for gallbladder cancer. Oncology. 2010; 79:168–73.
Article13. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012; 30:1934–40.
Article14. Ma N, Cheng H, Qin B, Zhong R, Wang B. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 2015; 15:615.
Article15. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424.
Article16. Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer. 2007; 110:572–80.17. Fong Y, Wagman L, Gonen M, Crawford J, Reed W, Swanson R, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg. 2006; 243:767–71.18. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article19. Yamanaka K, Hatano E, Kanai M, Tanaka S, Yamamoto K, Narita M, et al. A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol. 2014; 19:485–9.
Article20. Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, et al. SWOG S0809: a Phase II Intergroup Trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015; 33:2617–22.
Article21. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic factors of patients with advanced gallbladder carcinoma following aggressive surgical resection. J Gastrointest Surg. 2011; 15:1007–16.
Article22. Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011; 18:651–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reviewing the potential role of radiation therapy in gallbladder cancer: an update
- Effect of Early Adjuvant Chemotherapy on Survival of Advanced Gastric Cancer Patients: a Propensity Score-matched Analysis
- Survival Benefit of Adjuvant Chemotherapy in Patients with Pancreatic Ductal Adenocarcinoma Who Underwent Surgery Following Neoadjuvant FOLFIRINOX
- Multidisciplinary Therapeutic Approach to Gallbladder Cancer
- Immediate Breast Reconstruction Does Not Have a Clinically Significant Impact on Adjuvant Treatment Delay and Subsequent Survival Outcomes