J Clin Neurol.
2016 Apr;12(2):160-165. 10.3988/jcn.2016.12.2.160.
Therapeutic Outcomes and Prognostic Factors in Childhood Absence Epilepsy
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Korea University, Seoul, Korea. byeonagnes@naver.com
- KMID: 2354135
- DOI: http://doi.org/10.3988/jcn.2016.12.2.160
Abstract
- BACKGROUND AND PURPOSE
Childhood absence epilepsy (CAE) is one of the most common types of pediatric epilepsy. It is generally treated with ethosuximide (ESM), valproic acid (VPA), or lamotrigine (LTG), but the efficacy and adverse effects of these drugs remain controversial. This study compared initial therapy treatment outcomes, including VPA-LTG combination, and assessed clinical factors that may predict treatment response and prognosis.
METHODS
Sixty-seven patients with typical CAE were retrospectively enrolled at the Korea University Medical Center. We reviewed patients' clinical characteristics, including age of seizure onset, seizure-free interval, duration of seizure-free period, freedom from treatment failure, breakthrough seizures frequency, and electroencephalogram (EEG) findings.
RESULTS
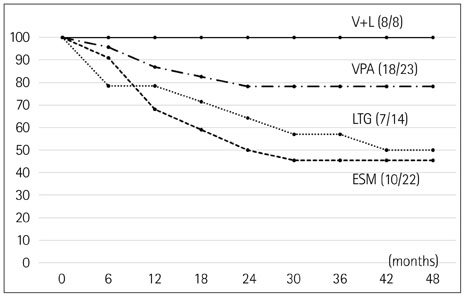
The age at seizure onset was 7.9±2.7 years (mean±SD), and follow-up duration was 4.4±3.7 years. Initially, 22 children were treated with ESM (32.8%), 23 with VPA (34.3%), 14 with LTG (20.9%), and 8 with VPA-LTG combination (11.9%). After 48 months of therapy, the rate of freedom from treatment failure was significantly higher for the VPA-LTG combination therapy than in the three monotherapy groups (p=0.012). The treatment dose administrated in the VPA-LTG combination group was less than that in the VPA and LTG monotherapy groups. The shorter interval to loss of 3-Hz spike-and-wave complexes and the presence of occipital intermittent rhythmic delta activity on EEG were significant factors predicting good treatment response.
CONCLUSIONS
This study showed that low-dose VPA-LTG combination therapy has a good efficacy and fewer side effects than other treatments, and it should thus be considered as a firstline therapy in absence epilepsy.
MeSH Terms
Figure
Reference
-
1. Panayiotopoulos CP. Treatment of typical absence seizures and related epileptic syndromes. Paediatr Drugs. 2001; 3:379–403.
Article2. Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005; 46:Suppl 9. 10–14.
Article3. Hirsch E, Panayiotopoulos CP. Childhood absence epilepsy and related syndromes. In : Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P, editors. Epileptic syndromes in infancy, childhood and adolescence. 4th ed. Montrouge: John Libbey Eurotext;2005. p. 315–335.4. Trinka E, Baumgartner S, Unterberger I, Unterrainer J, Luef G, Haberlandt E, et al. Long-term prognosis for childhood and juvenile absence epilepsy. J Neurol. 2004; 251:1235–1241.
Article5. Posner EB, Mohamed K, Marson AG. Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. Cochrane Database Syst Rev. 2005; (4):CD003032.
Article6. Lee JY, Yum MS, Kim EH, Lee EH, Ko TS. Lamotrigine as a first-line monotherapy in children with absence seizures. J Korean Child Neurol Soc. 2011; 19:142–149.7. Coppola G, Auricchio G, Federico R, Carotenuto M, Pascotto A. Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel-group study. Epilepsia. 2004; 45:1049–1053.
Article8. Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010; 362:790–799.
Article9. Coppola G, Licciardi F, Sciscio N, Russo F, Carotenuto M, Pascotto A. Lamotrigine as first-line drug in childhood absence epilepsy: a clinical and neurophysiological study. Brain Dev. 2004; 26:26–29.
Article10. Hughes JR. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav. 2009; 15:404–412.
Article11. Grosso S, Galimberti D, Vezzosi P, Farnetani M, Di Bartolo RM, Bazzotti S, et al. Childhood absence epilepsy: evolution and prognostic factors. Epilepsia. 2005; 46:1796–1801.
Article12. Wirrell EC. Natural history of absence epilepsy in children. Can J Neurol Sci. 2003; 30:184–188.
Article13. Matricardi S, Verrotti A, Chiarelli F, Cerminara C, Curatolo P. Current advances in childhood absence epilepsy. Pediatr Neurol. 2014; 50:205–212.
Article14. Mandelbaum DE, Burack GD, Bhise VV. Impact of antiepileptic drugs on cognition, behavior, and motor skills in children with newonset, idiopathic epilepsy. Epilepsy Behav. 2009; 16:341–344.
Article15. Guilhoto LM, Manreza ML, Yacubian EM. Occipital intermittent rhythmic delta activity in absence epilepsy. Arq Neuropsiquiatr. 2006; 64(2A):193–197.
Article16. Dlugos D, Shinnar S, Cnaan A, Hu F, Moshé S, Mizrahi E, et al. Pretreatment EEG in childhood absence epilepsy: associations with attention and treatment outcome. Neurology. 2013; 81:150–156.
Article17. Li Q, Luo C, Yang T, Yao Z, He L, Liu L, et al. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009; 87:160–168.
Article18. Wirrell EC, Camfield CS, Camfield PR, Gordon KE, Dooley JM. Long-term prognosis of typical childhood absence epilepsy: remission or progression to juvenile myoclonic epilepsy. Neurology. 1996; 47:912–918.
Article19. Posner EB, Mohamed K, Marson AG. A systematic review of treatment of typical absence seizures in children and adolescents with ethosuximide, sodium valproate or lamotrigine. Seizure. 2005; 14:117–122.
Article20. Wheless JW. Acute management of seizures in the syndromes of idiopathic generalized epilepsies. Epilepsia. 2003; 44:Suppl 2. 22–26.
Article21. Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia. 2013; 54:141–155.
Article22. Brodie MJ, Sills GJ. Combining antiepileptic drugs--rational polytherapy? Seizure. 2011; 20:369–375.
Article23. Pisani F, Oteri G, Russo MF, Di Perri R, Perucca E, Richens A. The efficacy of valproate-lamotrigine comedication in refractory complex partial seizures: evidence for a pharmacodynamic interaction. Epilepsia. 1999; 40:1141–1146.
Article24. Moeller JJ, Rahey SR, Sadler RM. Lamotrigine-valproic acid combination therapy for medically refractory epilepsy. Epilepsia. 2009; 50:475–479.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Factors for Absence Epilepsy in Childhood
- Concomitance of Childhood Absence Epilepsy and Benign Rolandic Spikes
- A Case of a Coincidence of Rolandic and Childhood Absence Epilepsy
- Predictive Factors for Valproate Treatment in Childhood Absence Epilepsy
- A Case of Atypical Benign Partial Epilepsy of Childhood Cured by Steroid