Clin Exp Otorhinolaryngol.
2016 Sep;9(3):244-251. 10.21053/ceo.2015.01011.
Radioprotective Effect of Epigallocatechin-3-Gallate on Salivary Gland Dysfunction After Radioiodine Ablation in a Murine Model
- Affiliations
-
- 1Department of Otorhinolaryngology, Inha University School of Medicine, Incheon, Korea. jylim@inha.ac.kr
- 2Department of Pathology, Inha University School of Medicine, Incheon, Korea.
- 3Department of Nuclear Medicine, National Cancer Center, Goyang, Korea.
- KMID: 2353623
- DOI: http://doi.org/10.21053/ceo.2015.01011
Abstract
OBJECTIVES
Radioiodine (RI) therapy is known to subject cellular components of salivary glands (SG) to oxidative stress leading to SG dysfunction. However, the protective effects of antioxidants on RI-induced SG damage have not been well investigated. The authors investigated the morphometric and functional effects of epigallocatechin-3-gallate (EGCG) administered prior to RI therapy and compared this with the effects of amifostine (a well-known antioxidant) in a murine model of RI sialadenitis.
METHODS
Four-week-old female C57BL/6 mice (n=48) were divided into four groups; a normal control group, a RI-treated group (0.01 mCi/g mouse, orally), an EGCG and RI-treated group, and an amifostine and RI-treated group. Animals in these groups were divided into 3 subgroups and euthanized at 15, 30, and 90 days post-RI treatment. Salivary flow rates and lag times were measured, and morphologic and histologic examinations and TUNEL (terminal deoxynucleotidyl transferase biotin-dUDP nick end labeling) assays were performed. Changes in salivary (99m)Tc pertechnetate uptake and excretion were followed by single-photon emission computed tomography.
RESULTS
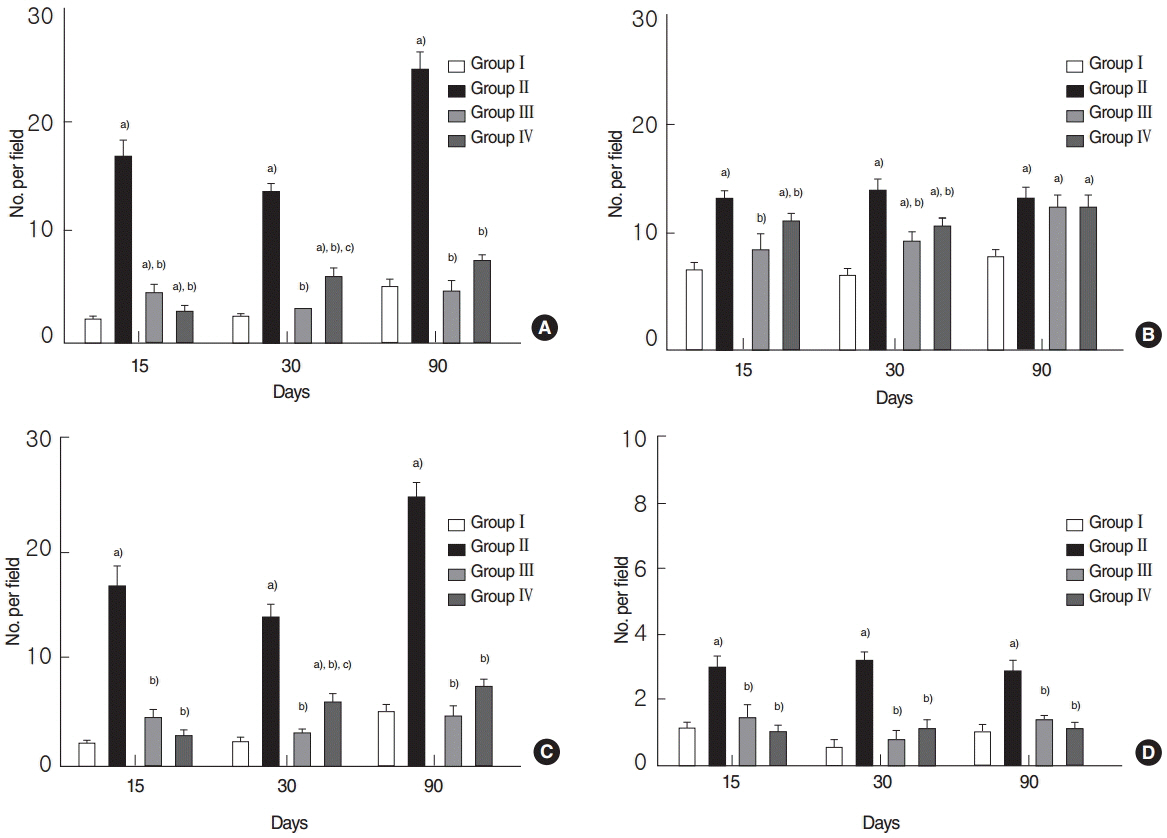
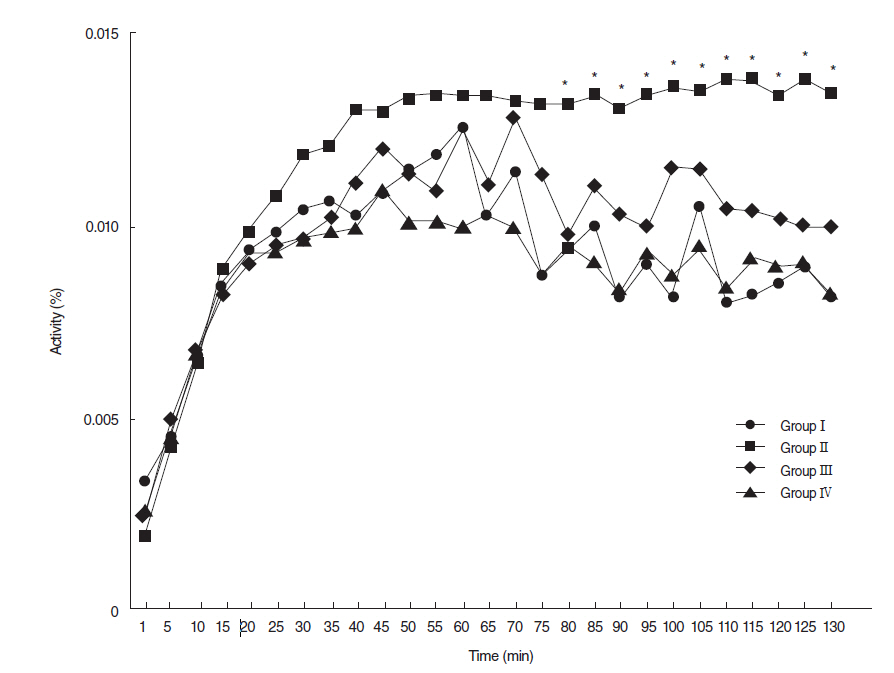
Salivary flow rates and lag times to salivation in the EGCG or amifostine groups were better than in the RI-treated group. Histologic examinations of SGs in the EGCG or amifostine group showed more mucin-rich parenchyma and less periductal fibrosis than in the RI-treated group. Fewer apoptotic cells were observed in acini, ducts, and among endothelial cells in the EGCG or amifostine group than in the RI group. In addition, patterns of (99m)Tc pertechnetate excretion were quite different in the EGCG or amifostine group than in the RI group.
CONCLUSION
EGCG supplementation before RI therapy could protect from RI-induced SG damage in a manner comparable to amifostine, and thus, offers a possible means of preventing SG damage by RI.
Keyword
MeSH Terms
-
Amifostine
Animals
Antioxidants
DNA Nucleotidylexotransferase
Endothelial Cells
Female
Fibrosis
Humans
In Situ Nick-End Labeling
Mice
Models, Animal
Oxidative Stress
Salivary Glands*
Salivation
Sialadenitis
Sodium Pertechnetate Tc 99m
Thyroid Neoplasms
Tomography, Emission-Computed
Tomography, Emission-Computed, Single-Photon
Amifostine
Antioxidants
DNA Nucleotidylexotransferase
Sodium Pertechnetate Tc 99m
Figure
Reference
-
1. Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003; Mar. 13(3):265–71.
Article2. Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009; Jul. 85(7):539–73.
Article3. Okumura H, Nasu M, Yosue T. Effects of amifostine administration prior to irradiation to the submandibular gland in mice: autoradiographic study using 3H-leucine. Okajimas Folia Anat Jpn. 2009; Feb. 85(4):151–60.
Article4. Joseph LJ, Bhartiya US, Raut YS, Hawaldar RW, Nayak Y, Pawar YP, et al. Radioprotective effect of Ocimum sanctum and amifostine on the salivary gland of rats after therapeutic radioiodine exposure. Cancer Biother Radiopharm. 2011; Dec. 26(6):737–43.
Article5. Ma C, Xie J, Jiang Z, Wang G, Zuo S. Does amifostine have radioprotective effects on salivary glands in high-dose radioactive iodine-treated differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010; Aug. 37(9):1778–85.
Article6. Ma C, Xie J, Chen Q, Wang G, Zuo S. Amifostine for salivary glands in high-dose radioactive iodine treated differentiated thyroid cancer. Cochrane Database Syst Rev. 2009; Oct. (4):CD007956.
Article7. Katiyar SK. Skin photoprotection by green tea: antioxidant and immunomodulatory effects. Curr Drug Targets Immune Endocr Metabol Disord. 2003; Sep. 3(3):234–42.
Article8. Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (-)-epigallocatechin-3-gallate. Oncogene. 2003; Feb. 22(7):1035–44.9. Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (-)- epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001; Feb. 22(2):287–94.10. Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; Jun. 71(6 Suppl):1698S–1702S.
Article11. Sueoka N, Suganuma M, Sueoka E, Okabe S, Matsuyama S, Imai K, et al. A new function of green tea: prevention of lifestyle-related diseases. Ann N Y Acad Sci. 2001; Apr. 928:274–80.
Article12. Hsu S, Dickinson DP, Qin H, Lapp C, Lapp D, Borke J, et al. Inhibition of autoantigen expression by (-)-epigallocatechin-3-gallate (the major constituent of green tea) in normal human cells. J Pharmacol Exp Ther. 2005; Nov. 315(2):805–11.
Article13. Vanhove C, Defrise M, Franken PR, Everaert H, Deconinck F, Bossuyt A. Interest of the ordered subsets expectation maximization (OSEM) algorithm in pinhole single-photon emission tomography reconstruction: a phantom study. Eur J Nucl Med. 2000; Feb. 27(2):140–6.
Article14. Defrise M, Vanhove C, Nuyts J. Perturbative refinement of the geometric calibration in pinhole SPECT. IEEE Trans Med Imaging. 2008; Feb. 27(2):204–14.
Article15. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015; May. 136(9):2187–95.
Article16. Levy O, De la Vieja A, Carrasco N. The Na+/I- symporter (NIS): recent advances. J Bioenerg Biomembr. 1998; Apr. 30(2):195–206.17. Van Nostrand D. Sialoadenitis secondary to 131I therapy for well-differentiated thyroid cancer. Oral Dis. 2011; Mar. 17(2):154–61.18. La Perle KM, Kim DC, Hall NC, Bobbey A, Shen DH, Nagy RS, et al. Modulation of sodium/iodide symporter expression in the salivary gland. Thyroid. 2013; Aug. 23(8):1029–36.
Article19. Dorr RT. Radioprotectants: pharmacology and clinical applications of amifostine. Semin Radiat Oncol. 1998; Oct. 8(4 Suppl 1):10–3.20. Hensley ML, Schuchter LM, Lindley C, Meropol NJ, Cohen GI, Broder G, et al. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J Clin Oncol. 1999; Oct. 17(10):3333–55.21. Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010; Aug. 18(8):1061–79.
Article22. Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007; Oct. 12(19-20):794–805.
Article23. Kim SJ, Choi HY, Kim IJ, Kim YK, Jun S, Nam HY, et al. Limited cytoprotective effects of amifostine in high-dose radioactive iodine 131-treated well-differentiated thyroid cancer patients: analysis of quantitative salivary scan. Thyroid. 2008; Mar. 18(3):325–31.
Article24. Noaparast Z, Hosseinimehr SJ. Radioprotective agents for the prevention of side effects induced by radioiodine-131 therapy. Future Oncol. 2013; Aug. 9(8):1145–59.
Article25. Dickinson D, DeRossi S, Yu H, Thomas C, Kragor C, Paquin B, et al. Epigallocatechin-3-gallate modulates anti-oxidant defense enzyme expression in murine submandibular and pancreatic exocrine gland cells and human HSG cells. Autoimmunity. 2014; May. 47(3):177–84.
Article26. Saito K, Mori S, Date F, Ono M. Epigallocatechin gallate inhibits oxidative stress-induced DNA damage and apoptosis in MRL-Fas(lpr) mice with autoimmune sialadenitis via upregulation of heme oxygenase-1 and Bcl-2. Autoimmunity. 2014; Feb. 47(1):13–22.27. Kutta H, Kampen U, Sagowski C, Brenner W, Bohuslavizki KH, Paulsen F. Amifostine is a potent radioprotector of salivary glands in radioiodine therapy. Structural and ultrastructural findings. Strahlenther Onkol. 2005; Apr. 181(4):237–45.28. Choi JS, Park IS, Kim SK, Lim JY, Kim YM. Morphometric and functional changes of salivary gland dysfunction after radioactive iodine ablation in a murine model. Thyroid. 2013; Nov. 23(11):1445–51.
Article29. Choi JS, Park IS, Kim SK, Lim JY, Kim YM. Analysis of age-related changes in the functional morphologies of salivary glands in mice. Arch Oral Biol. 2013; Nov. 58(11):1635–42.
Article30. Yamamoto T, Hsu S, Lewis J, Wataha J, Dickinson D, Singh B, et al. Green tea polyphenol causes differential oxidative environments in tumor versus normal epithelial cells. J Pharmacol Exp Ther. 2003; Oct. 307(1):230–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Salivary Gland Radioiodine Retention on Post-ablation 131I Scintigraphy as a Predictor of Salivary Gland Dysfunction in Patients with Differentiated Thyroid Carcinoma
- Clinical Outcome of Parotid Gland Massage for Preventing Parotid Gland Dysfunction in Patients Treated with Radioiodine Therapy for Differentiated Thyroid Cancer: a Prospective Longitudinal Follow-Up Study
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- Effect of Epigallocatechin Gallate on Viability of Kudoa septempunctata
- Preliminary study on the efficacy of xerostomia treatment with sialocentesis targeting thyroid disease patients given radioiodine therapy