Yonsei Med J.
2015 Jan;56(1):82-88. 10.3349/ymj.2015.56.1.82.
Immunohistochemical Detection of p53 Expression in Patients with Preoperative Chemoradiation for Rectal Cancer: Association with Prognosis
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. lwy555@skku.edu
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2352792
- DOI: http://doi.org/10.3349/ymj.2015.56.1.82
Abstract
- PURPOSE
The expression of p53 in patients with rectal cancer who underwent preoperative chemoradiationand and its potential prognostic significance were evaluated.
MATERIALS AND METHODS
p53 expression was examined using immunohistochemistry in pathologic specimens from 210 rectal cancer patients with preoperative chemoradiotherapy and radical surgery. All patients were classified into two groups according to the p53 expression: low p53 (<50% nuclear staining) and high p53 (> or =50%) groups.
RESULTS
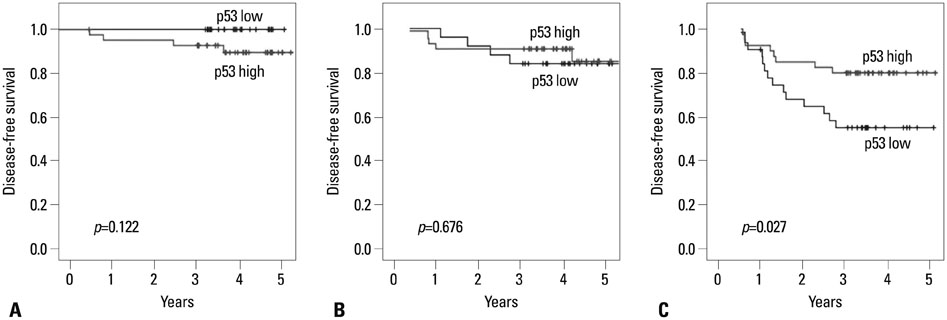
p53 expression was significantly associated with tumor location from the anal verge (p=0.036). In univariate analysis, p53 expression was not associated with disease-free survival (p=0.118) or local recurrence-free survival (p=0.089). Multivariate analysis showed that tumor distance from the anal verge (p=0.006), ypN category (p=0.011), and perineural invasion (p=0.048) were independent predictors of disease-free survival; tumor distance from the anal verge was the only independent predictor of local recurrence-free survival. When the p53 groups were subdivided according to ypTNM category, disease-free survival differed significantly in patients with ypN+ disease (p=0.027) only.
CONCLUSION
Expression of p53 in pathologic specimens as measured by immunohistochemical methods may have a significant prognostic impact on survival in patients with ypN+ rectal cancer with preoperative chemoradiotherapy. However, it was not an independent predictor of recurrence or survival.
Keyword
MeSH Terms
-
Adult
Aged
*Chemoradiotherapy
Disease-Free Survival
Female
Humans
Immunohistochemistry
Male
Middle Aged
Multivariate Analysis
Neoplasm Recurrence, Local/pathology
Neoplasm Staging
*Preoperative Care
Prognosis
Rectal Neoplasms/diagnosis/*metabolism/surgery/*therapy
Tumor Suppressor Protein p53/analysis/immunology/*metabolism
Tumor Suppressor Protein p53
Figure
Reference
-
1. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009; 27:5124–5130.
Article2. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009; 373:811–820.
Article3. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740.
Article4. García-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003; 46:298–304.
Article5. Huh JW, Park YA, Jung EJ, Lee KY, Sohn SK. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J Am Coll Surg. 2008; 207:7–12.
Article6. Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U Jr, Silva E Sousa AH Jr, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005; 9:90–99.
Article7. Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989; 342:705–708.
Article8. Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001; 344:1196–1206.
Article9. Spitz FR, Giacco GG, Hess K, Larry L, Rich TA, Janjan N, et al. p53 immunohistochemical staining predicts residual disease after chemoradiation in patients with high-risk rectal cancer. Clin Cancer Res. 1997; 3:1685–1690.10. Schwandner O, Schiedeck TH, Bruch HP, Duchrow M, Windhoevel U, Broll R. p53 and Bcl-2 as significant predictors of recurrence and survival in rectal cancer. Eur J Cancer. 2000; 36:348–356.
Article11. Jakob C, Liersch T, Meyer W, Becker H, Baretton GB, Aust DE. Predictive value of Ki67 and p53 in locally advanced rectal cancer: correlation with thymidylate synthase and histopathological tumor regression after neoadjuvant 5-FU-based chemoradiotherapy. World J Gastroenterol. 2008; 14:1060–1066.
Article12. Saw RP, Morgan M, Koorey D, Painter D, Findlay M, Stevens G, et al. p53, deleted in colorectal cancer gene, and thymidylate synthase as predictors of histopathologic response and survival in low, locally advanced rectal cancer treated with preoperative adjuvant therapy. Dis Colon Rectum. 2003; 46:192–202.
Article13. Kim HR, Kim HC, Yun HR, Kim SH, Park CK, Cho YB, et al. An alternative pathway in colorectal carcinogenesis based on the mismatch repair system and p53 expression in Korean patients with sporadic colorectal cancer. Ann Surg Oncol. 2013; 20:4031–4040.
Article14. Park CH, Kim HC, Cho YB, Yun SH, Lee WY, Park YS, et al. Predicting tumor response after preoperative chemoradiation using clinical parameters in rectal cancer. World J Gastroenterol. 2011; 17:5310–5316.
Article15. Lee H, Park HC, Park W, Choi DH, Kim YI, Park YS, et al. Negative impact of pretreatment anemia on local control after neoadjuvant chemoradiotherapy and surgery for rectal cancer. Radiat Oncol J. 2012; 30:117–123.
Article16. Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994; 73:2680–2686.
Article17. Hendriks Y, Franken P, Dierssen JW, De Leeuw W, Wijnen J, Dreef E, et al. Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol. 2003; 162:469–477.
Article18. Rashid A, Zahurak M, Goodman SN, Hamilton SR. Genetic epidemiology of mutated K-ras proto-oncogene, altered suppressor genes, and microsatellite instability in colorectal adenomas. Gut. 1999; 44:826–833.
Article19. Huh JW, Lee JH, Kim HR. Expression of p16, p53, and Ki-67 in colorectal adenocarcinoma: a study of 356 surgically resected cases. Hepatogastroenterology. 2010; 57:734–740.20. Bouzourene H, Gervaz P, Cerottini JP, Benhattar J, Chaubert P, Saraga E, et al. p53 and Ki-ras as prognostic factors for Dukes’ stage B colorectal cancer. Eur J Cancer. 2000; 36:1008–1015.
Article21. Zeng ZS, Sarkis AS, Zhang ZF, Klimstra DS, Charytonowicz E, Guillem JG, et al. p53 nuclear overexpression: an independent predictor of survival in lymph node--positive colorectal cancer patients. J Clin Oncol. 1994; 12:2043–2050.
Article22. Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC, Bunn PA Jr, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res. 1998; 58:1149–1158.23. Soong R, Grieu F, Robbins P, Dix B, Chen D, Parsons R, et al. p53 alterations are associated with improved prognosis in distal colonic carcinomas. Clin Cancer Res. 1997; 3:1405–1411.24. Morgan M, Koorey D, Painter D, Findlay M, Newland R, Chapuis P, et al. p53 and DCC immunohistochemistry in curative rectal cancer surgery. Int J Colorectal Dis. 2003; 18:188–195.
Article25. Birkenkamp-Demtroder K, Olesen SH, Sørensen FB, Laurberg S, Laiho P, Aaltonen LA, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005; 54:374–384.
Article26. Kapiteijn E, Liefers GJ, Los LC, Kranenbarg EK, Hermans J, Tollenaar RA, et al. Mechanisms of oncogenesis in colon versus rectal cancer. J Pathol. 2001; 195:171–178.
Article27. Hilska M, Collan YU, O Laine VJ, Kössi J, Hirsimäki P, Laato M, et al. The significance of tumor markers for proliferation and apoptosis in predicting survival in colorectal cancer. Dis Colon Rectum. 2005; 48:2197–2208.
Article28. Leahy DT, Salman R, Mulcahy H, Sheahan K, O'Donoghue DP, Parfrey NA. Prognostic significance of p53 abnormalities in colorectal carcinoma detected by PCR-SSCP and immunohistochemical analysis. J Pathol. 1996; 180:364–370.
Article29. Watson AJ, Merritt AJ, Jones LS, Askew JN, Anderson E, Becciolini A, et al. Evidence of reciprocity of bcl-2 and p53 expression in human colorectal adenomas and carcinomas. Br J Cancer. 1996; 73:889–895.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Significance of Cathepsin D and p53 Expressionin Locally Advanced Rectal Cancer
- Significance of p53 Overexpression in the Metastasis of Colorectal Cancer
- p53, Bcl-2 and Ki-67 Expression according to Tumor Response after Concurrent Chemoradiation Treatment for Advanced Rectal Cancer
- Pathological Analysis of Tumor Response after Preoperative Chemoradiation Therapy for Advanced Rectal Cancer
- Disadvantages of Preoperative Chemoradiation in Rectal Cancer