Korean J Ophthalmol.
2014 Dec;28(6):466-472. 10.3341/kjo.2014.28.6.466.
Intravitreal Anti-vascular Endothelial Growth Factor for Typical Exudative Age-related Macular Degeneration in Eyes with Good Baseline Visual Acuity
- Affiliations
-
- 1Department of Ophthalmology, Konyang University College of Medicine, Daejeon, Korea.
- 2Department of Ophthalmology, Kim's Eye Hospital, Konyang University College of Medicine, Seoul, Korea. kimoph@gmail.com
- KMID: 2345823
- DOI: http://doi.org/10.3341/kjo.2014.28.6.466
Abstract
- PURPOSE
To investigate 12-month treatment outcomes of anti-vascular endothelial growth factor therapy in eyes with typical exudative age-related macular degeneration with good baseline visual acuity.
METHODS
This retrospective observational case series included 18 eyes (18 patients) with typical exudative age-related macular degeneration with a baseline best-corrected visual acuity of 20 / 25 or better. Patients were treated with anti-vascular endothelial growth factor monotherapy during the 12-month follow-up period. Baseline visual acuity and central foveal thickness were compared to the values at 12 months.
RESULTS
Patients received an average of 4.4 +/- 1.3 intravitreal anti-vascular endothelial growth factor injections. The mean logarithm of minimum angle of resolution visual acuity was 0.08 +/- 0.04, 0.08 +/- 0.07, 0.12 +/- 0.09, and 0.16 +/- 0.11 at baseline, three months, six months, and 12 months, respectively. Visual acuity at 12 months was significantly worse than the baseline value at diagnosis (p = 0.017), and the mean central foveal thickness at the defined time points was 270.2 +/- 55.6, 204.4 +/- 25.4, 230.1 +/- 56.3, and 216.8 +/- 48.7 microm, respectively. The central foveal thickness at 12 months was significantly less than the baseline value at diagnosis (p = 0.042).
CONCLUSIONS
Deterioration in visual acuity was noted in eyes with typical exudative age-related macular degeneration with good baseline visual acuity, suggesting the need for close patient monitoring and prompt treatment even in patients with good baseline visual acuity.
Keyword
MeSH Terms
-
Aged
Angiogenesis Inhibitors/*therapeutic use
Bevacizumab/therapeutic use
Choroidal Neovascularization/*drug therapy/physiopathology
Female
Fluorescein Angiography
Humans
Intravitreal Injections
Male
Middle Aged
Ranibizumab/therapeutic use
Retrospective Studies
Tomography, Optical Coherence
Vascular Endothelial Growth Factor A/antagonists & inhibitors
Visual Acuity/*physiology
Wet Macular Degeneration/*drug therapy/physiopathology
Angiogenesis Inhibitors
Bevacizumab
Ranibizumab
Vascular Endothelial Growth Factor A
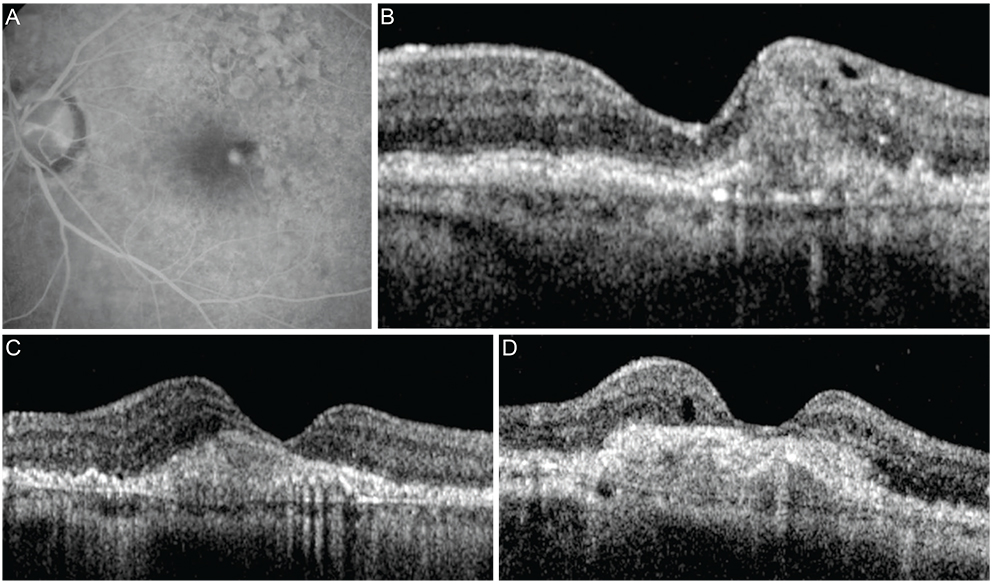
Figure
Reference
-
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.2. Lee SY, Kim JG, Joe SG, et al. The therapeutic effects of bevacizumab in patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2008; 22:92–99.3. Cho HJ, Baek JS, Lee DW, et al. Short-term effectiveness of intravitreal bevacizumab vs. ranibizumab injections for patients with polypoidal choroidal vasculopathy. Korean J Ophthalmol. 2012; 26:157–162.4. Mones J, Biarnes M, Trindade F, Casaroli-Marano R. FUSION regimen: ranibizumab in treatment-naïve patients with exudative age-related macular degeneration and relatively good baseline visual acuity. Graefes Arch Clin Exp Ophthalmol. 2012; 250:1737–1744.5. Takahashi M, Sato T, Kishi S. Intravitreal bevacizumab for age-related macular degeneration with good visual acuity. Jpn J Ophthalmol. 2010; 54:565–570.6. Axer-Siegel R, Bor E, Bourla DH, et al. Intravitreal bevacizumab treatment for exudative age-related macular degeneration with good visual acuity. Retina. 2012; 32:1811–1820.7. Mori R, Yuzawa M, Akaza E, Haruyama M. Treatment results at 1 year of ranibizumab therapy for polypoidal choroidal vasculopathy in eyes with good visual acuity. Jpn J Ophthalmol. 2013; 57:365–371.8. Shona O, Gupta B, Vemala R, Sivaprasad S. Visual acuity outcomes in ranibizumab-treated neovascular age-related macular degeneration; stratified by baseline vision. Clin Experiment Ophthalmol. 2011; 39:5–8.9. Matsumiya W, Honda S, Kusuhara S, et al. Effectiveness of intravitreal ranibizumab in exudative age-related macular degeneration (AMD): comparison between typical neovascular AMD and polypoidal choroidal vasculopathy over a 1 year follow-up. BMC Ophthalmol. 2013; 13:10.10. Yamashiro K, Tomita K, Tsujikawa A, et al. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol. 2012; 154:125–136.11. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–583.12. Liu TY, Shah AR, Del Priore LV. Progression of lesion size in untreated eyes with exudative age-related macular degeneration: a meta-analysis using Lineweaver-Burk plots. JAMA Ophthalmol. 2013; 131:335–340.13. Arias L, Ruiz-Moreno JM, Gomez-Ulla F, et al. A 1-year retrospective review of ranibizumab for naive nonsubfoveal choroidal neovascularization secondary to age-related macular degeneration. Retina. 2009; 29:1444–1449.14. You JY, Chung H, Kim HC. Evaluation of changes in choroidal neovascularization secondary to age-related macular degeneration after anti-VEGF therapy using spectral domain optical coherence tomography. Curr Eye Res. 2012; 37:438–445.15. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.16. Dadgostar H, Ventura AA, Chung JY, et al. Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology. 2009; 116:1740–1747.17. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Early Visual Responses to Anti-Vascular Endothelial Growth Factor in Age-related Macular Degeneration
- Clinical Outcomes of Eyes with Submacular Hemorrhage Secondary to Age-related Macular Degeneration Treated with Anti-vascular Endothelial Growth Factor
- Treatment of Exudative Age-Related Macular Degeneration
- The Effect of Intravitreal Bevacizumab Injection in Wet Age-Related Macular Degeneration Patients with Cataract Surgery
- Long-Term Longitudinal Choroidal Thickness Changes after Intravitreal Anti-Vascular Endothelial Growth Factor Injections in Patients with Neovascular Age-Related Macular Degeneration