Cancer Res Treat.
2016 Jul;48(3):1092-1101. 10.4143/crt.2015.316.
Systemic Treatments for Metastatic Renal Cell Carcinoma: 10-Year Experience of Immunotherapy and Targeted Therapy
- Affiliations
-
- 1Department of Urology, Center for Prostate Cancer, Research Institute and Hospital of National Cancer Center, Goyang, Korea. cjs5225@ncc.re.kr
- 2Department of Pathology, Center for Prostate Cancer, Research Institute and Hospital of National Cancer Center, Goyang, Korea.
- 3Department of Radiology, Center for Prostate Cancer, Research Institute and Hospital of National Cancer Center, Goyang, Korea.
- KMID: 2344083
- DOI: http://doi.org/10.4143/crt.2015.316
Abstract
- PURPOSE
The purpose of this study is to compare the outcomes of first-line systemic targeted therapy (TT) and immunotherapy (IT) in patients with metastatic renal cell carcinoma (mRCC).
MATERIALS AND METHODS
This study was a retrospective review of the data of 262 patients treated with systemic IT or TT with tyrosine kinase inhibitors between 2003 and 2013. The objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) were assessed using Response Evaluation Criteria in Solid Tumor ver. 1.0 criteria and the Kaplan-Meier method with log-rank test.
RESULTS
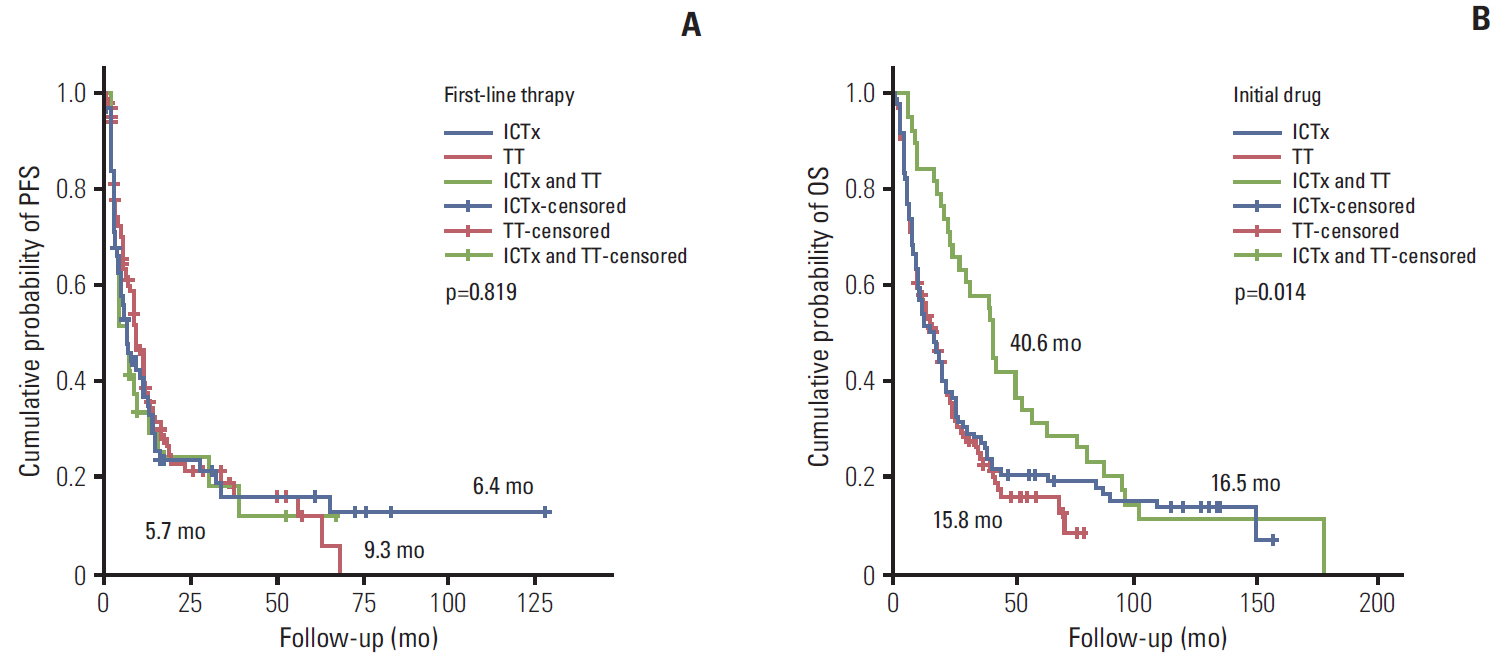
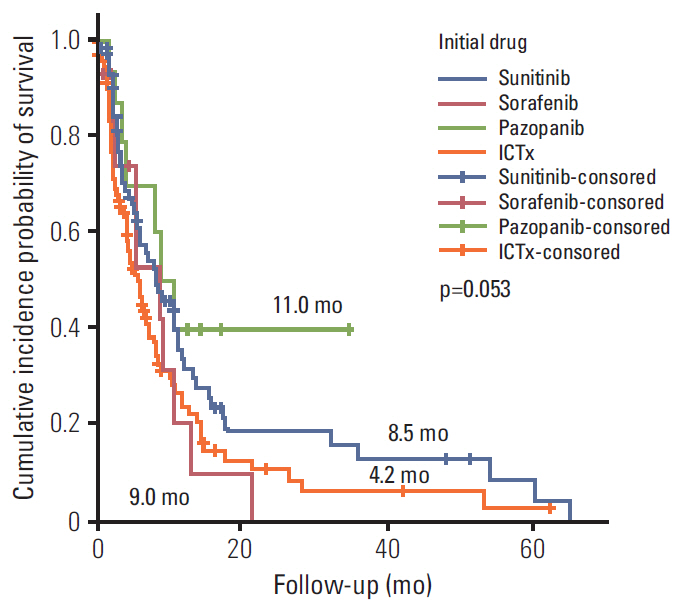
During the median 4.3-month treatment and the 24-month follow-up period, the ORR/PFS/OS of the overall first-line and second-line therapy were 41.9%/8.1 months/16.8 months and 27.5%/6.5 months/15.3 months, respectively. The first-line TT/IT/sequential IT had a PFS of 9.3/6.4/5.7 months and an OS of 15.8/16.5/40.6 months (all p < 0.05). The second-line of TT/IT had a PFS of 7.1/2.1 months (both p < 0.05) and an OS of 16.6/8.6 months (p=0.636), respectively. Pazopanib provided the best median PFS of 11.0 months (p < 0.001) and a quadruple IT regimen had a superior PFS (p=0.522). For OS, sequential treatment with IT and TT was superior compared to treatment with either IT or TT alone (40.6/16.5/15.8 months, p=0.014). The prognosis according to the Memorial Sloan Kettering Cancer Center model showed that favorable/intermediate/poor risk groups had a PFS of 8.5/10.4/2.3 months, and an OS of 43.1/20.4/5.6 months, respectively. The prognosis calculated using the Heng model showed that the favorable/intermediate/poor risk groups had a PFS of 9.2/3.9/2.7 months, and an OS of 32.4/16.5/6.1months, respectively (all p < 0.001).
CONCLUSION
In patients with mRCC, TT provided a better PFS and OS compared with IT.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Survival prognoses of Heng intermediate-risk patients with metastatic renal cell carcinoma treated with immunotherapy or targeted therapy: A real-world, single-center retrospective study
Sung Han Kim, Dong-Eun Lee, Jae Young Joung, Ho Kyung Seo, Kang Hyun Lee, Jinsoo Chung
Investig Clin Urol. 2020;61(2):146-157. doi: 10.4111/icu.2020.61.2.146.The Experience of Uncertainty in Cancer Patients Undergoing Chemotherapy
Yoon Sun Kim, Young Sook Tae, Keum Hee Nam, Heui Yeoung Kim
Asian Oncol Nurs. 2018;18(3):115-126. doi: 10.5388/aon.2018.18.3.115.
Reference
-
References
1. Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009; 7:618–30.2. Oudard S, George D, Medioni J, Motzer R. Treatment options in renal cell carcinoma: past, present and future. Ann Oncol. 2007; 18 Suppl 10:x25–31.
Article3. Fossa SD, Raabe N, Moe B. Recombinant interferon-alpha with or without vinblastine in metastatic renal carcinoma: results of a randomised phase II study. Br J Urol. 1989; 64:468–71.4. Pyrhonen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999; 17:2859–67.5. Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000; 6 Suppl 1:S55–7.6. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995; 13:688–96.
Article7. Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012; 53:217–28.
Article8. Lee JH, Chang SG, Jeon SH, Min GE, Yoo KH. Comparative analysis between immunochemotherapy and target therapy for metastatic renal cell carcinoma: overview of treatment-related adverse events and the dropout rate in Korea. Korean J Urol. 2010; 51:379–85.
Article9. Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009; 27:5794–9.
Article10. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002; 20:289–96.
Article11. Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000; 163:408–17.
Article12. McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res. 2007; 13(2 Pt 2):716s–20s.
Article13. Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009; 27:1280–9.
Article14. Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010; 28:475–80.
Article15. Desai AA, Stadler WM. Novel kinase inhibitors in renal cell carcinoma: progressive development of static agents. Curr Urol Rep. 2006; 7:16–22.
Article16. Akaza H, Naito S, Ueno N, Aoki K, Houzawa H, Pitman Lowenthal S, et al. Real-world use of sunitinib in Japanese patients with advanced renal cell carcinoma: efficacy, safety and biomarker analyses in 1689 consecutive patients. Jpn J Clin Oncol. 2015; 45:576–83.
Article17. Akaza H, Oya M, Iijima M, Hyodo I, Gemma A, Itoh H, et al. A large-scale prospective registration study of the safety and efficacy of sorafenib tosylate in unresectable or metastatic renal cell carcinoma in Japan: results of over 3200 consecutive cases in post-marketing all-patient surveillance. Jpn J Clin Oncol. 2015; 45:953–62.
Article18. Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer. 2015; 113:12–9.19. Albiges L, Oudard S, Negrier S, Caty A, Gravis G, Joly F, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012; 30:482–7.
Article20. Johannsen M, Staehler M, Ohlmann CH, Florcken A, Schmittel A, Otto T, et al. Outcome of treatment discontinuation in patients with metastatic renal cell carcinoma and no evidence of disease following targeted therapy with or without metastasectomy. Ann Oncol. 2011; 22:657–63.
Article21. Matrana MR, Bathala T, Campbell MT, Duran C, Shetty A, Teegavarapu P, et al. Outcomes of unselected patients with metastatic clear-cell renal cell carcinoma treated with front-line pazopanib therapy followed by vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKI) or mammalian target of rapamycin inhibitors (mTORi): a single institution experience. BJU Int. 2015; Nov. 17. [Epub]. http://dx.doi.org/10.1111/bju.13374.22. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article23. Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004; 54:78–93.
Article24. Sadeghi S, Albiges L, Wood LS, Black SL, Gilligan TD, Dreicer R, et al. Cessation of vascular endothelial growth factor-targeted therapy in patients with metastatic renal cell carcinoma: feasibility and clinical outcome. Cancer. 2012; 118:3277–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunotherapy for Renal Cell Carcinoma
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy
- Novel immunotherapy in metastatic renal cell carcinoma
- Cytoreductive nephrectomy in the age of immunotherapy-based combination treatment
- Metastatic Renal Cell Carcinoma in the Paranasal Sinus: A Case Report and Literature Review