J Korean Ophthalmol Soc.
2014 Aug;55(8):1208-1212.
Effect of Bevacizumab and Ranibizumab on the Expression of eNOS in Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
Abstract
- PURPOSE
To compare the effects of bevacizumab and ranibizumab on the expression of eNOS in cultured human trabecular meshwork cells (HTMC).
METHODS
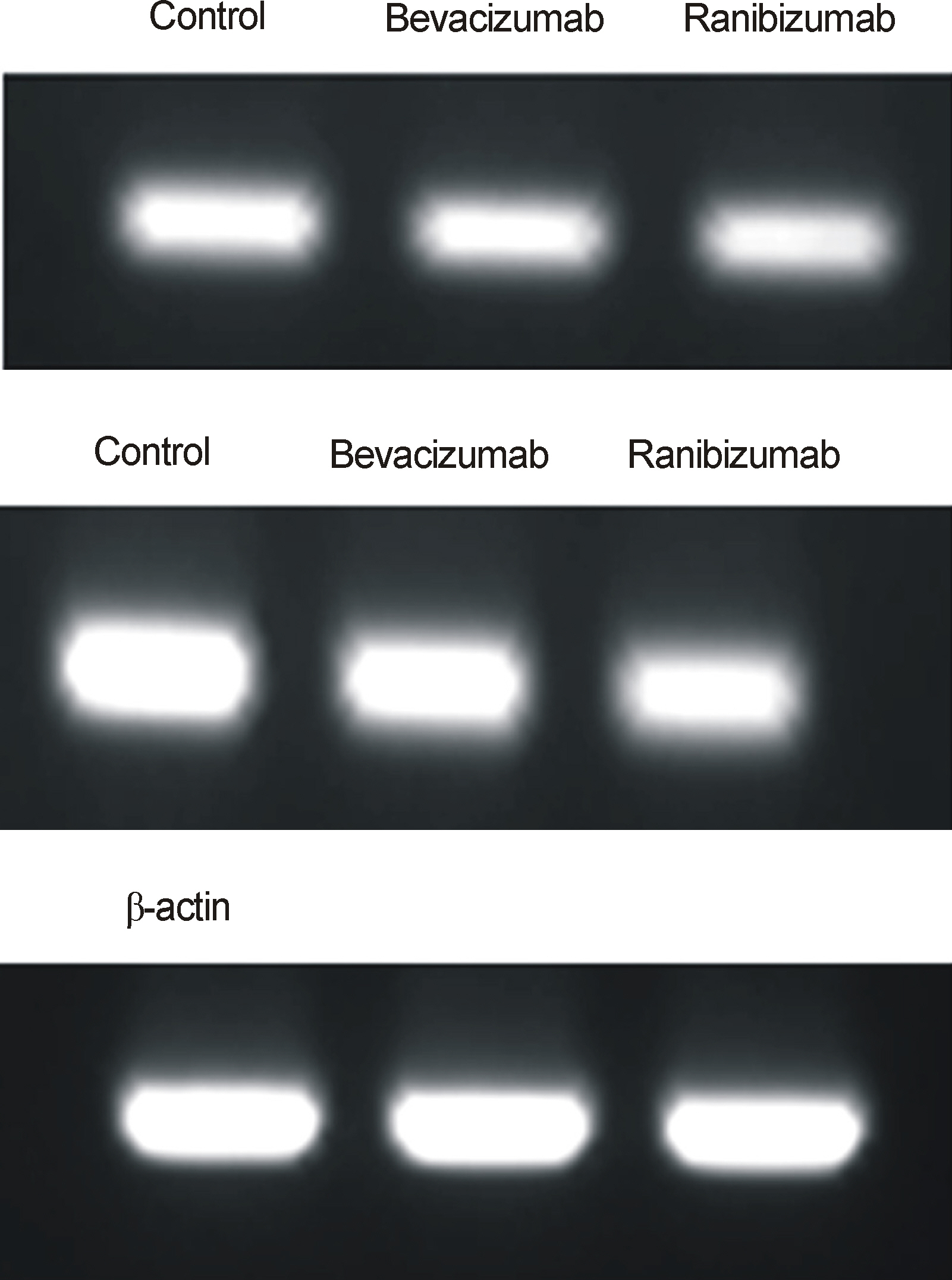
Primarily cultured HTMC were exposed to 0.5 mg/mL bevacizumab and ranibizumab using 1% serum-containing media for 30 minutes. Expression of eNOS mRNA was assessed with RT-PCR. Additionally, after exposure to 20 ng/mL of vascular endothelial growth factor (VEGF) and 0.5 mg/mL bevacizumab and ranibizumab, the production of nitric oxide was assessed with Griess assay.
RESULTS
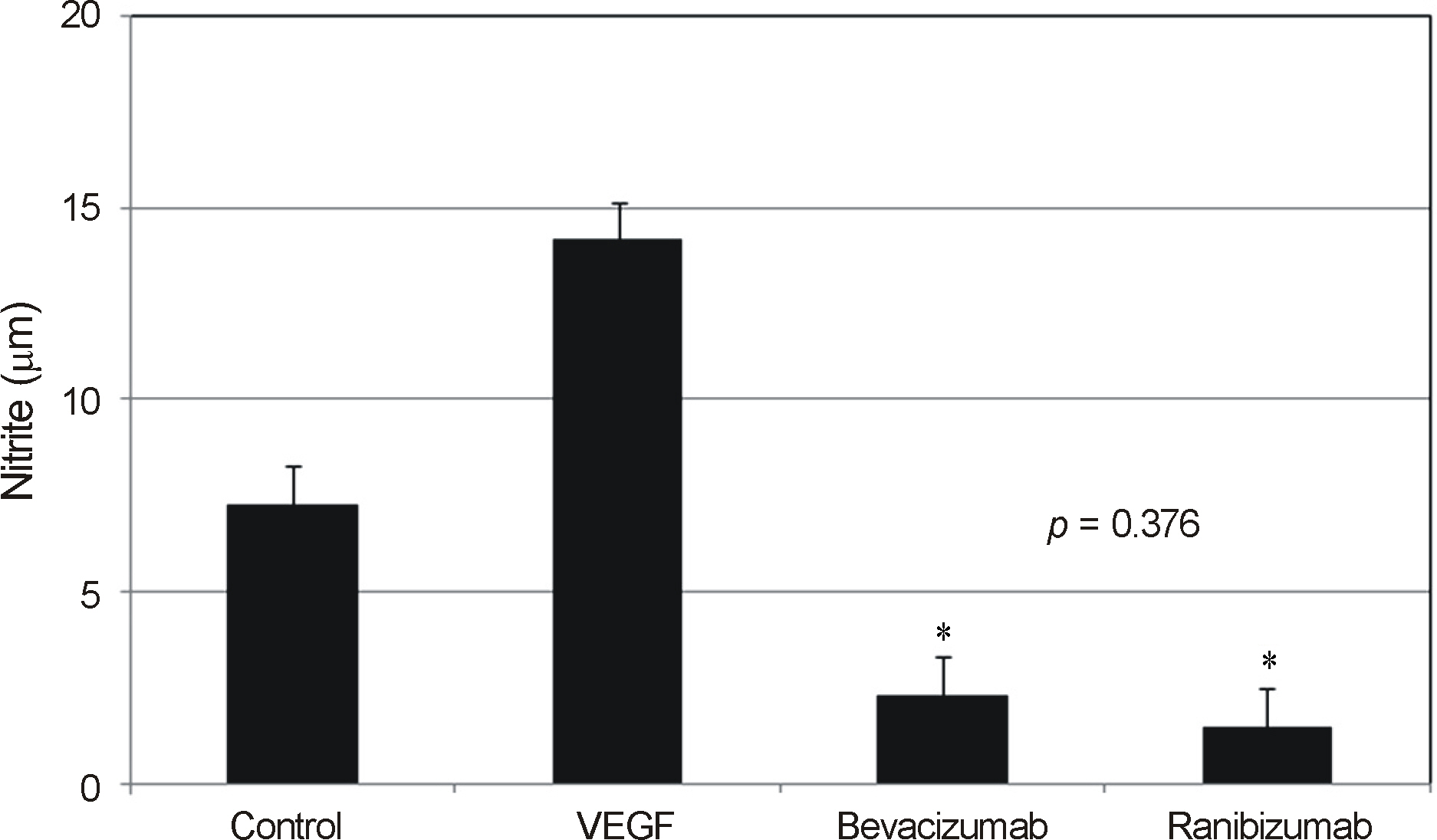
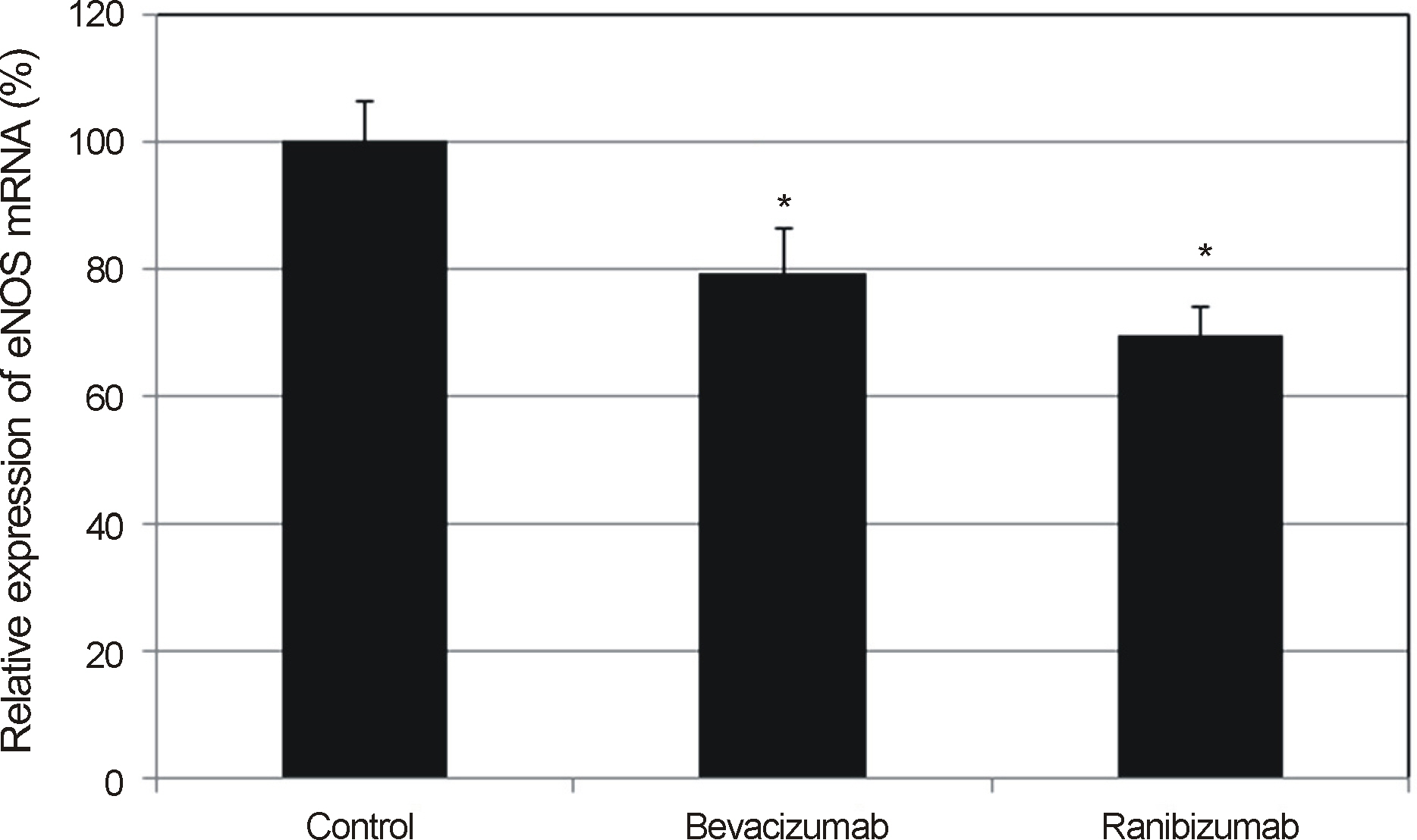
VEGF increased the production of nitric oxide in HTMC. Bevacizumab and ranibizumab decreased the expression of eNOS mRNA and production of nitric oxide (p < 0.05) in HTMC. The decrease in eNOS mRNA expression and production of nitric oxide was not significant between bevacizumab and ranibizumab (p > 0.05).
CONCLUSIONS
In HTMC, both bevacizumab and ranibizumab decreased the expression of eNOS mRNA with no significant difference observed between the two drugs.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine Rev. 1997; 18:4–25.
Article2. Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999; 41:773–80.3. Dulak J, Jozkowicz A, Dembinska-Kiec A, et al. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arteioscler Thromb Vasc Biol. 2000; 20:659–66.
Article4. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011; 95:1111–4.
Article5. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.6. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eye. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.
Article7. Meyer P, Champion C, Schlotzer-Schrehardt U, et al. Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.
Article8. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–20.9. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–5.10. Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.11. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–8.
Article12. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.13. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.14. Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998; 83:832–40.15. Xia P, Aiello LP, Ishii H, et al. Characterization of vascular endothelial growth factor's effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest. 1996; 98:2018–26.
Article16. Yoon SH, Kim JW. A study of the pathway of nitric oxide production by nitroglycerin in trabecular meshwork cells. J Korean Ophthalmol Soc. 2013; 54:1429–34.
Article17. Spitzer MS, Yoeruek E, Sierra A, et al. Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1837–42.
Article18. Kahook MY, Ammar DA. In vitro effects of antivascular endothelial growth factors on cultured human trabecular meshwork cells. J Glaucoma. 2010; 19:437–41.
Article19. Kim SH, Kim JW. Effect of bevacizumab on survival and production of nitric oxide in trabecular meshwork cells. J Korean Ophthalmol Sci. 2009; 50:1404–8.
Article20. Kernt M, Welge-Lussen U, Yu A, et al. Bevacizumab is not toxic to human anterior- and posterior-segment cultured cells. Ophthalmologe. 2007; 104:965–71.21. Kahook MY, Kimura AE, Wong LJ, et al. Sustained elevation of intraocular pressure associated with intravitreal bevacizumab injections. Ophthalmic Surg Lasers Imaging. 2009; 40:293–5.22. Bakri SJ, McCannel CA, Edwards AO, Moshfeghi DM. Persistent ocular hypertension following intravitreal ranibizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:955–8.23. Ladas JG, Yu F, Loo R, et al. Relationship between aqueous humor protein level and outflow facility in patients with uveitis. Invest Ophthalmol Vis Sci. 2001; 42:2584–8.24. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58:99–105.
Article25. Wang RF, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Bevacizumab on Survival and Production of Nitric Oxide in Trabecular Meshwork Cells
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- The Effects of Topical Carbonic Anhydrase Inhibitors on Nitric Oxide Production in Trabecular Meshwork Cells
- A Study of the Pathway of Nitric Oxide Production by Nitroglycerin in Trabecular Meshwork Cells
- Effect of Valproic Acid on Nitric Oxide and Nitric Oxide Synthase in Trabecular Meshwork Cell