J Korean Ophthalmol Soc.
2018 Jun;59(6):543-548. 10.3341/jkos.2018.59.6.543.
Effect of Valproic Acid on Nitric Oxide and Nitric Oxide Synthase in Trabecular Meshwork Cell
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- 2Hyundai Eye Clinic, Kumi, Korea.
- KMID: 2413817
- DOI: http://doi.org/10.3341/jkos.2018.59.6.543
Abstract
- PURPOSE
To investigate the effects of valproic acid on the production of nitric oxide (NO) and expression of endothelial nitric oxide synthase (eNOS) in cultured human trabecular meshwork cells (HTMC).
METHODS
Primarily cultured HTMC were exposed to 0.25, 0.5, and 1.0 mM valproic acid for 6, 12, and 24 hours. Expression of eNOS mRNA was assessed with Reverse transcription-polymerase chain reaction, and production of NO was assessed with Griess assay. Cellular survival was assessed with the 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay.
RESULTS
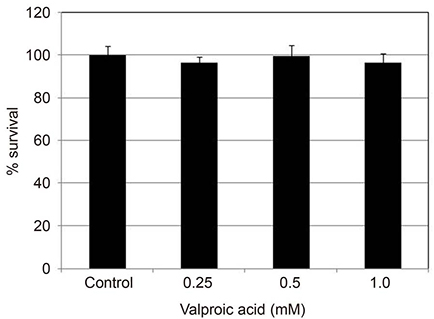
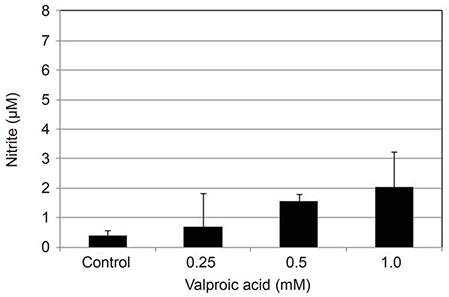
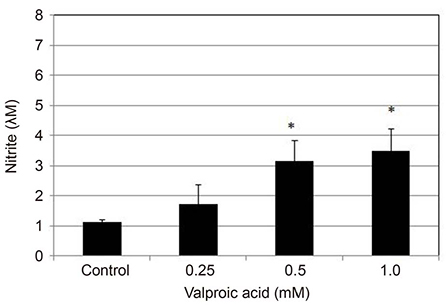
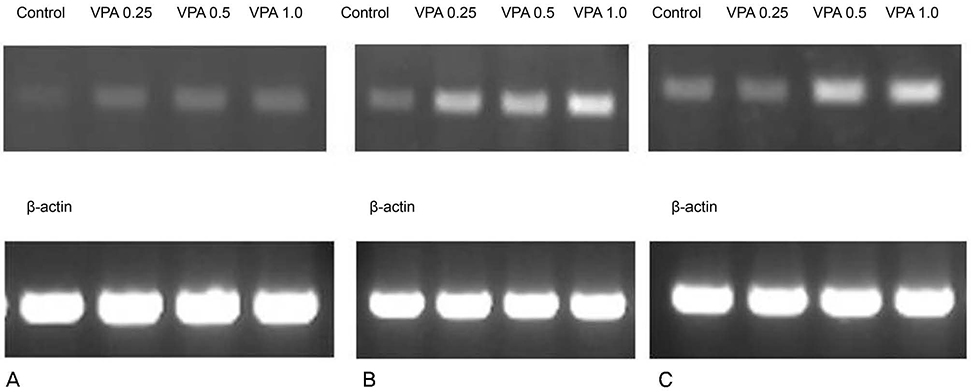
Valproic acid at concentrations of 0.25, 0.5, 1.0 mM did not affect the cellular survival of HTMC significantly after exposure for 24 hours. Valproic acid increased NO production in a dose- and time-dependent manner. Also, valproic acid increased the degree of eNOS mRNA expression in a dose-dependent manner in HTMC.
CONCLUSIONS
Valproic acid increases production of NO and expression of eNOS mRNA in HTMC. Thus, valproic acid might increase aqueous outflow through the trabecular meshwork.
MeSH Terms
Figure
Reference
-
1. Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002; 16:669–694.2. Monti B, Polazzi E, Contestabile A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol. 2009; 2:95–109.
Article3. Michaelis M, Michaelis UR, Fleming I, et al. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004; 65:520–527.
Article4. Hyndman KA, Ho DH, Sega MF, Pollock JS. Histone deacetylase 1 reduces NO production in endothelial cells via lysine deacetylation of NO synthase 3. Am J Physiol Heart Circ Physiol. 2014; 307:H803–H809.
Article5. Cho DH, Park JH, Joo Lee E, et al. Valproic acid increases NO production via the SH-PTP1-CDK5-eNOS-Ser(116) signaling cascade in endothelial cells and mice. Free Radic Biol Med. 2014; 76:96–106.
Article6. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–579.
Article7. Rohen JW, Lütjen-drecoll E, Flügel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–692.8. Wiederholt M, Dörschner N, Groth J. Effect of diuretics, channel modulators and signal interceptors on contractility of the trabecular meshwork. Ophthalmologica. 1997; 211:153–160.
Article9. Wiederholt M, Stumpff F. The trabecular meshwork and aqueous humor reabsorption. In : Civan MM, editor. Current Topics in Membranes. The Eye's Aqueous Humor: From Secretion To Glaucoma. 1st ed. San Diego: Academic Press;1998. v. 45. chap. 7.10. Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35:2515–2520.11. Behar-Cohen FF, Goureau O, D'Hermies F, Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest Ophthalmol Vis Sci. 1996; 37:1711–1715.12. Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008; 294:C1378–C1386.
Article13. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article14. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–138.15. Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000; 20:347–350.
Article16. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–1049.17. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–478.18. Göttlicher M, Minucci S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001; 20:6969–6978.19. Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001; 276:36734–36741.
Article20. Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase-dependent and -independent apoptotic signaling pathway. Leuk Res. 2002; 26:495–502.21. Phillips A, Bullock T, Plant N. Sodium valproate induces apoptosis in the rat hepatoma cell line, FaO. Toxicology. 2003; 192:219–227.
Article22. Tang R, Faussat AM, Majdak P, et al. Valproic acid inhibits proliferation and induces apoptosis in acute myeloid leukemia cells expressing P-gp and MRP1. Leukemia. 2004; 18:1246–1251.
Article23. Witt D, Burfeind P, von Hardenberg S, et al. Valproic acid inhibits the proliferation of cancer cells by re-expressing cyclin D2. Carcinogenesis. 2013; 34:1115–1124.
Article24. Biermann J, Grieshaber P, Goebel U, et al. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010; 51:526–534.
Article25. Alsarraf O, Fan J, Dahrouj M, et al. Acetylation preserves retinal ganglion cell structure and function in a chronic model of ocular hypertension. Invest Ophthalmol Vis Sci. 2014; 55:7486–7493.
Article26. Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998; 273:24266–24271.
Article27. Bose P, Dai Y, Grant S. Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther. 2014; 143:323–336.
Article28. Zhang Z, Qin X, Zhao X, et al. Valproic acid regulates antioxidant enzymes and prevents ischemia/reperfusion injury in the rat retina. Curr Eye Res. 2012; 37:429–437.
Article29. Kimura A, Namekata K, Guo X, et al. Valproic acid prevents NMDA-induced retinal ganglion cell death via stimulation of neuronal TrkB receptor signaling. Am J Pathol. 2015; 185:756–764.
Article30. Kimura A, Guo X, Noro T, et al. Valproic acid prevents retinal degeneration in a murine model of normal tension glaucoma. Neurosci Lett. 2015; 588:108–113.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Mitomycin C on the Proliferation and Nitric Oxide Production in the Cultured Trabecular Meshwork Cells
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- Effect of Nitric Oxide on the Permeability of Trabecular Meshwork Cell Monolayer
- Nitric Oxide Synthase Inhibition Alters Extracellular Glutamate Concentration after Global Cerebral Ischemia
- Effect of beta-adrenergics on the Survival and Production of Nitric Oxide in the Cultured Trabecular Meshwork Cells