J Korean Ophthalmol Soc.
2010 Aug;51(8):1113-1120.
Effect of Preservative-free Artificial Eye Drop on Human Corneal Epithelial Cell in vitro
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Medical Research Institute, Pusan National University Hospital, Busan, Korea. jongsool@pusan.ac.kr
- 2Department of Ophthalmology, St. Mary's Hospital, Busan, Korea.
Abstract
- PURPOSE
To investigate the biologic effects of preservative-free artificial tear drops on cultured human corneal epithelial cells in vitro.
METHODS
Efficacies of the preservative-free artificial tear drops-Kynex(R), Hyalein Mini 0.3%(R), and Refresh Plus(R)-were evaluated using the MTT assay. Cell damage was determined using the lactate dehydrogenase (LDH) assay. Cellular proliferation was determined using a migration and wound-healing assay. The ingredients of the drugs were analyzed. Apoptotic response was evaluated with flow cytometric analysis.
RESULTS
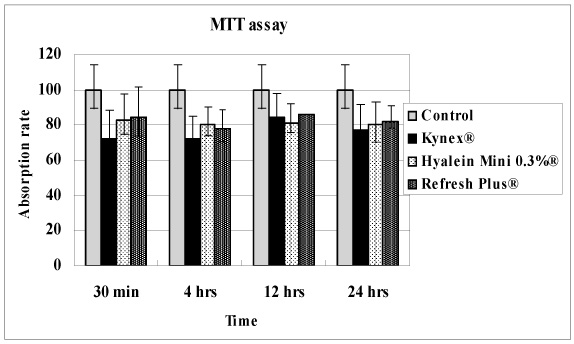
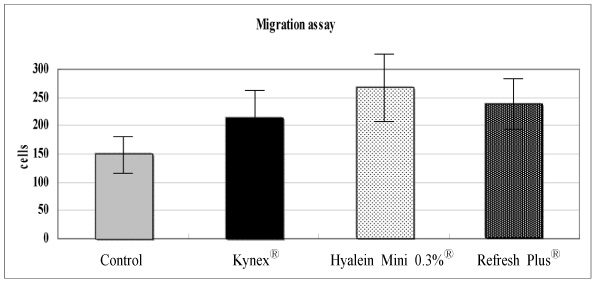
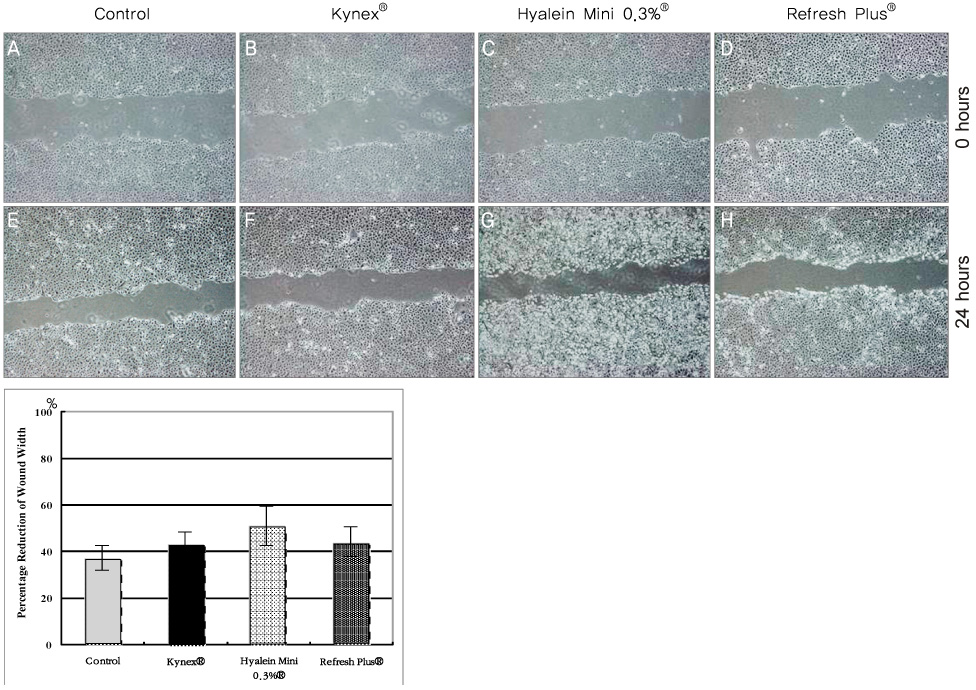
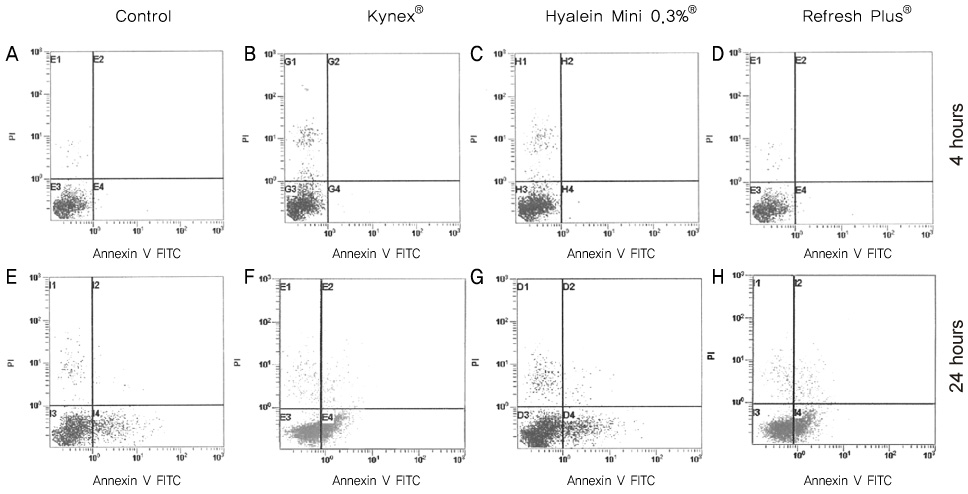
Metabolic activity of the corneal epithelial cells showed similar activity to that of the control. Cellular migration and proliferation also were not significantly different between the preservative-free artificial tear drop groups and the control. The LDH titers tended to increase for up to 24 hours after exposure to the preservative-free artificial tear drops, but there was no significant difference in LDH titers between the control groups and the artificial tear drop-treated groups. Apoptosis and necrosis were observed using flow cytometry at 24 hours in all groups. The electrolyte levels, pHs and osmolarities of the three drugs were not significantly different.
CONCLUSIONS
The clinically available preservative-free artificial tear drops Kynex(R), Hyalein Mini 0.3%(R), and Refresh Plus(R) had no significant toxic effects on corneal epithelial cells and thus can be used safely.
MeSH Terms
Figure
Reference
-
1. Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjuncival epithelial cells. Clin Experiment Ophthalmol. 2008. 36:553–559.2. Tonjum AM. Effects of benzalkonium chloride upon the corneal epithelium studies with scanning electron microscopy. Acta Ophthalmol. 1975. 53:358–366.3. Burstein NL. Coneal cytotoxictiy of topically applied drugs, vehicles and preservatives. Surv Opthalmol. 1980. 25:15–30.4. Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Opthlamol Vis Sci. 1980. 19:308–313.5. Collin HB, Grabsch BE. The effect of ophthalmic preservatives on the healing rate of the rabbit corneal epithelium after keratectomy. Am J Opthalmol Physiol Opt. 1982. 59:215–222.6. Mosmann TJ. Rapid colorimetric assay for cellular growth and sruvival: Application to proliferation and cytotoxic assays. J Immunolo Methods. 1983. 65:55–63.7. Garrett Q, Simmons PA, Xu S, et al. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007. 48:1559–1567.8. Berdy GJ, Abelson MB, Smith LM, George MA. Preservative-free artificial tear preparations; assessment of corneal epithelial toxic effects. Arch Opthalmol. 1992. 110:528–532.9. Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Opthalmol. 2006. 244:109–112.10. Miyauchi S, Sugiyama T, Machida A, et al. The effect of sodium hyaluronate on the migration of rabbit corneal epithelium. J Ocul Pharmacol. 1990. 6:91–99.11. Nishida T, Nakamura M, Mishima H, Otori T. Hyaluronate stimulate corneal epithelial migration. Exp Eye Res. 1991. 53:753–758.12. Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Opthalmol. 2004. 88:821–825.13. Chung JH. Experimental corneal alkali wound healing. Acta Ophthalmol Suppl. 1988. 187:1–35.14. Chung JH, Fargerholm P, Lindstrom B. Hyaluronate in healing of corneal alkali wound in the rabbit. Exp Eye Res. 1989. 48:569–576.15. Garrett Q, Xu S, Simmons PA, et al. Carboxymethyl cellulose stimulate rabbit corneal epithelial wound healing. Curr Eye Res. 2008. 33:567–573.16. Garrett Q, Simmons PA, Xu S, et al. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Opthalmol Vis Sci. 2007. 48:1559–1567.17. Ahee JA, Kaufman SC, Samuel MA, et al. Decreased incidence of epithelial defects during laser in situ keratomileusis using intraoperative nonpreserved carboxymethylcellulose sodium 0.5% solution. J Cataract Refract Surg. 2002. 28:1651–1654.18. Adler R. Mechanism of photoreceptor death in retinal degeneration. From the cell biology of the 1990s to the ophthalmology of the 21st century. Arch Ophthalmol. 1996. 114:79–83.19. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995. 267:1456–1462.20. Wee WR, Wang XW, McDonnell PJ. Effect of artificial tears on cultured keratocytes in vitro. Cornea. 1995. 109:860–863.21. Park YS. Kang DH, editor. Physiology of body fluid. Physiology. 2000. 5th ed. Seoul: Sin-Kwang publishing & printing;chap.5.22. Freshney RI. The culture environment: Substrate, gas phase, medium, and temparature. 1987. 2nd ed. New York: Wiley-Liss;70–71.23. Kim K. Sung HK, Kim KH, editors. Acid-base balance. Physiology. 1996. Seoul: Eui-hak publishing & printing;326–327.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Preservative-Free Healon Eye Drop on Human Corneal Epithelial Cell in Vitro
- Long-Term Effect of Preservative-Free Sodium Hyaluronate Eye Drop on Human Corneal Epithelial Cell
- Effect of Artificial Tears Used in Contact Lens-wearing Eyes on Human Corneal Epithelial Cells in Vitro
- Effect of the Preservative Benzalkonium Chloride in Prostaglandin Analogues on Corneal Sensitivity
- The Effect of 5% Serum Albumin on Intractable Corneal Epithelial Keratitis: a Case Series and Literature Review