J Korean Orthop Assoc.
2009 Aug;44(4):436-441.
Prevention of New Vertebral Fractures after Treatment with Risedronate, Alendronate or Calcium Carbonate in Patients with Osteoporotic Compression Fracture Treated with Cement Augmentation
- Affiliations
-
- 1Department of Orthopaedic Surgery, College of Medicine, Yonsei University, Seoul, Korea. haksunkim@yuhs.ac
Abstract
- PURPOSE
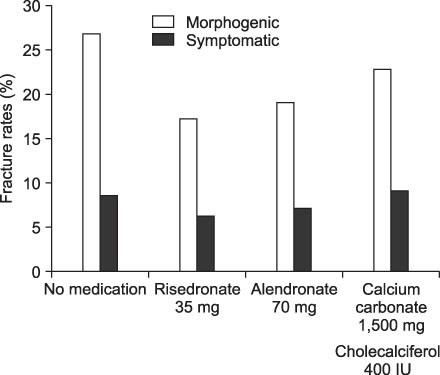
To evaluate the rate of new fractures of the spine after risedronate, alendronate or calcium carbonate in patients who had vertebroplasty or kyphoplasty due to compression fracture. MATERIALS AND METHODS: We studied 292 patients with osteoporotic compression fractures who had received vertebroplasty or kyophoplasty between June 2003 and October 2007. Of these, 199 were evaluated for new fractures of the spine after treatment with risedronate, alendronate or calcium carbonate. Patients (n=199) were assigned to 1 of 4 groups: No treatment (n=71), risendronate (n=64), alendronate (n=42) or calcium carbonate group (n=22). RESULTS: New fractures of the spine were morphogenically found in 19 patients (26.8%) in the no treatment group, in 11 (17.2%) in the risendronate group, in 8 (19.1%) in the alendronate group, in 5 (22.8%) in the calcium carbonate group. Symptomatically, they were found in 6 patients (8.5%) in the no treatment group, in 4 (6.3%) in the risendronate group, in 3 patients (7.1%) in the alendronate group, and in 2 patients (9.1%) in the calcium carbonate group. CONCLUSION: At one year follow up none of the differences between groups in new fracture rates of the spine were statistically significant.
MeSH Terms
Figure
Reference
-
1. Al-Azzawi F, Hart DM, Lindsay R. Long term effect of oestrogen replacement therapy on bone mass as measured by dual photon absorptiometry. Br Med J (Clin Res Ed). 1987. 294:1261–1262.
Article2. Lufkin EG, Wahner HW, O'Fallon WM, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med. 1992. 117:1–9.
Article3. Overgaard K, Hansen MA, Jensen SB, Christiansen C. Effect of calcitonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ. 1992. 305:556–561.4. Pak CY, Sakhaee K, Adams-Huet B, Piziak V, Peterson RD, Poindexter JR. Treatment of postmenopausal osteoporosis with slow release sodium fluoride: final report of a randomized controlled trial. Ann Intern Med. 1995. 123:401–408.5. Riggs BL, Seeman E, Hodgson SF, Taves DR, O'Fallon WM. Effect of the fluoride/calcium regimen on vertebral fracture occurrence in postmenopausal osteoporosis. N Engl J Med. 1982. 306:446–450.
Article6. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995. 333:1437–1443.
Article7. Storm T, Thamsborg G, Steiniche T, Genant HK, Sorenson OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med. 1990. 322:1265–1271.
Article8. Watts NB, Harris ST, Genant HK, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990. 323:73–79.
Article9. Cooper C, Melton LJ. Vertebral fractures. BMJ. 1992. 304:793–794.
Article10. Black DM, Cummings SR, Karpf DB, et al. Randomised trail of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996. 348:1535–1541.11. Tilyard MW, Spears GFS, Thomson J, Dovey S. Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med. 1992. 326:357–362.
Article12. Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys. 2000. 373:231–241.
Article13. Devogelaer JP, Broll H, Correa-Rotter R, et al. Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis. Bone. 1996. 18:141–150.
Article14. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. pean Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000. 15:1006–1013.15. Reginster JY, Minne HW, Sørensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000. 11:83–91.
Article16. Kushida K, Fukunaga M, Kishimoto H, et al. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab. 2004. 22:469–478.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of New Fractures after Treatment with Alendronate or Raloxifene in Patients with Osteoporotic Compression Fracture Treated with Cement Augmentation
- Percutaneous Vertebral Augmentation for the Treatment of Osteoporotic Spinal Fractures
- Risk Factors for Subsequent Fracture after Osteoporotic Vertebral Compression Fracture
- Cement Leakage into Disc after Kyphoplasty: Does It Increases the Risk of New Adjacent Vertebral Fractures?
- The Proper Volume and Distribution of Cement Augmentation on Percutaneous Vertebroplasty