J Periodontal Implant Sci.
2015 Apr;45(2):46-55. 10.5051/jpis.2015.45.2.46.
Effectiveness of biphasic calcium phosphate block bone substitutes processed using a modified extrusion method in rabbit calvarial defects
- Affiliations
-
- 1Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea.
- 2Department of Periodontology, Research Institute for Periodontal Regeneration, College of Dentistry, Yonsei University, Seoul, Korea. shchoi726@yuhs.ac
- 3School of Materials Science & Engineering, Yeungnam University, Gyeongsan, Korea.
- KMID: 2329655
- DOI: http://doi.org/10.5051/jpis.2015.45.2.46
Abstract
- PURPOSE
This study evaluated the mechanical and structural properties of biphasic calcium phosphate (BCP) blocks processed using a modified extrusion method, and assessed their in vivo effectiveness using a rabbit calvarial defect model.
METHODS
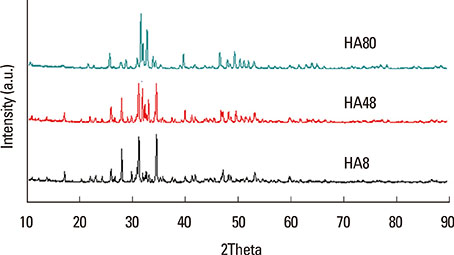
BCP blocks with three distinct ratios of hydroxyapatite (HA):tricalcium phosphate (TCP) were produced using a modified extrusion method:HA8 (8%:92%), HA48 (48%:52%), and HA80 (80%:20%). The blocks were examined using scanning electron microscopy, X-ray diffractometry, and a universal test machine. Four circular defects 8 mm in diameter were made in 12 rabbits. One defect in each animal served as a control, and the other three defects received the BCP blocks. The rabbits were sacrificed at either two weeks (n=6) or eight weeks (n=6) postoperatively.
RESULTS
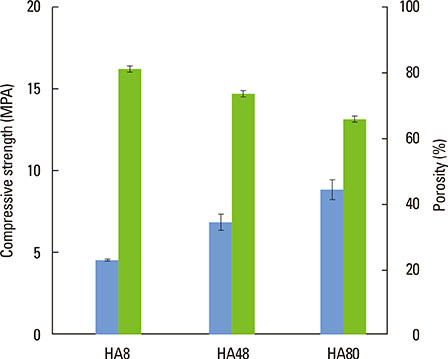
The pore size, porosity, and compressive strength of the three types of bone block were 140-170 microm, >70%, and 4-9 MPa, respectively. Histologic and histomorphometric observations revealed that the augmented space was well maintained, but limited bone formation was observed around the defect base and defect margins. No significant differences were found in the amount of new bone formation, graft material resorption, or bone infiltration among the three types of BCP block at either of the postoperative healing points.
CONCLUSIONS
Block bone substitutes with three distinct compositions (i.e., HA:TCP ratios) processed by a modified extrusion method exhibited limited osteoconductive potency, but excellent space-maintaining capability. Further investigations are required to improve the processing method.
MeSH Terms
Figure
Cited by 2 articles
-
Late-term healing in an augmented sinus with different ratios of biphasic calcium phosphate: a pilot study using a rabbit sinus model
Hyun-Chang Lim, Ji-Youn Hong, Jung-Seok Lee, Ui-Won Jung, Seong-Ho Choi
J Periodontal Implant Sci. 2016;46(1):57-69. doi: 10.5051/jpis.2016.46.1.57.Biomimetic characteristics of mussel adhesive protein-loaded collagen membrane in guided bone regeneration of rabbit calvarial defects
Woong-Kyu Song, Joo-Hyun Kang, Jae-Kook Cha, Jung-Seok Lee, Jeong-Won Paik, Ui-Won Jung, Byung-Hoon Kim, Seong-Ho Choi
J Periodontal Implant Sci. 2018;48(5):305-316. doi: 10.5051/jpis.2018.48.5.305.
Reference
-
1. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007; 22:Suppl. 49–70.2. Aloy-Prósper A, Maestre-Ferrin L, Peñarrocha-Oltra D, Peñarrocha-Diago M. Bone regeneration using particulate grafts: an update. Med Oral Patol Oral Cir Bucal. 2011; 16:e210–e214.3. Tinti C, Parma-Benfenati S. Clinical classification of bone defects concerning the placement of dental implants. Int J Periodontics Restorative Dent. 2003; 23:147–155.
Article4. Park SH, Brooks SL, Oh TJ, Wang HL. Effect of ridge morphology on guided bone regeneration outcome: conventional tomographic study. J Periodontol. 2009; 80:1231–1236.
Article5. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007; 78:377–396.
Article6. Hwang JW, Park JS, Lee JS, Jung UW, Kim CS, Cho KS, et al. Comparative evaluation of three calcium phosphate synthetic block bone graft materials for bone regeneration in rabbit calvaria. J Biomed Mater Res B Appl Biomater. 2012; 100:2044–2052.
Article7. Petrungaro PS, Amar S. Localized ridge augmentation with allogenic block grafts prior to implant placement: case reports and histologic evaluations. Implant Dent. 2005; 14:139–148.
Article8. Torres J, Tamimi F, Alkhraisat MH, Prados-Frutos JC, Rastikerdar E, Gbureck U, et al. Vertical bone augmentation with 3D-synthetic monetite blocks in the rabbit calvaria. J Clin Periodontol. 2011; 38:1147–1153.
Article9. Xuan F, Lee CU, Son JS, Fang Y, Jeong SM, Choi BH. Vertical ridge augmentation using xenogenous bone blocks: a comparison between the flap and tunneling procedures. J Oral Maxillofac Surg. 2014; 72:1660–1670.
Article10. Kim JW, Jung IH, Lee KI, Jung UW, Kim CS, Choi SH, et al. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block loaded with rhBMP-2 in vertical bone augmentation model of a rabbit calvarium. J Biomed Mater Res A. 2012; 100:3304–3313.
Article11. Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials. 1998; 19:1473–1478.
Article12. Studart AR, Gonzenbach UT, Tervoort E, Gauckler LJ. Processing routes to macroporous ceramics: a review. J Am Ceram Soc. 2006; 89:1771–1789.
Article13. Hing KA. Bioceramic bone graft substitutes: influence of porosity and chemistry. Int J Appl Ceram Tec. 2005; 2:184–199.
Article14. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, et al. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:298–306.
Article15. Lim S, Chun S, Yang D, Kim S. Comparison Study of Porous Calcium Phosphate Blocks Prepared by Piston and Screw Type Extruders for Bone Scaffold. Tissue Eng Regen Med. 2012; 9:51–55.
Article16. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005; 26:5474–5491.
Article17. Chvapil M, Holusa R, Kliment K, Stoll M. Some chemical and biological characteristics of a new collagen-polymer compound material. J Biomed Mater Res. 1969; 3:315–332.
Article18. Welsh RP, Pilliar RM, Macnab I. Surgical implants. The role of surface porosity in fixation to bone and acrylic. J Bone Joint Surg Am. 1971; 53:963–977.19. Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001; 83-A:Suppl 1. S105–S115.
Article20. Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997; 121:317–324.
Article21. Hing KA, Best SM, Tanner KE, Bonfield W, Revell PA. Mediation of bone ingrowth in porous hydroxyapatite bone graft substitutes. J Biomed Mater Res A. 2004; 68:187–200.
Article22. Li S, De Wijn JR, Li J, Layrolle P, De Groot K. Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng. 2003; 9:535–548.
Article23. Hing KA, Best SM, Bonfield W. Characterization of porous hydroxyapatite. J Mater Sci Mater Med. 1999; 10:135–145.24. Bohner M, Baumgart F. Theoretical model to determine the effects of geometrical factors on the resorption of calcium phosphate bone substitutes. Biomaterials. 2004; 25:3569–3582.
Article25. Bignon A, Chouteau J, Chevalier J, Fantozzi G, Carret JP, Chavassieux P, et al. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med. 2003; 14:1089–1097.
Article26. Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, et al. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010; 40:180–187.
Article27. Yang C, Unursaikhan O, Lee JS, Jung UW, Kim CS, Choi SH. Osteoconductivity and biodegradation of synthetic bone substitutes with different tricalcium phosphate contents in rabbits. J Biomed Mater Res B Appl Biomater. 2014; 102:80–88.
Article28. Yip I, Ma L, Mattheos N, Dard M, Lang NP. Defect healing with various bone substitutes. Clin Oral Implants Res. 2015; 26:606–614.
Article29. Hassanein AH, Couto RA, Kurek KC, Rogers GF, Mulliken JB, Greene AK. Experimental comparison of cranial particulate bone graft, rhBMP-2, and split cranial bone graft for inlay cranioplasty. Cleft Palate Craniofac J. 2013; 50:358–362.
Article30. Daculsi G, Passuti N, Martin S, Deudon C, Legeros RZ, Raher S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990; 24:379–396.
Article31. Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998; 19:133–139.
Article32. Lu JX, Flautre B, Anselme K, Hardouin P, Gallur A, Descamps M, et al. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J Mater Sci Mater Med. 1999; 10:111–120.33. Kirchhoff M, Lenz S, Henkel KO, Frerich B, Holzhüter G, Radefeldt S, et al. Lateral augmentation of the mandible in minipigs with a synthetic nanostructured hydroxyapatite block. J Biomed Mater Res B Appl Biomater. 2011; 96:342–350.
Article34. Eggli PS, Müller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin Orthop Relat Res. 1988; 127–138.35. Jovanovic SA, Schenk RK, Orsini M, Kenney EB. Supracrestal bone formation around dental implants: an experimental dog study. Int J Oral Maxillofac Implants. 1995; 10:23–31.36. Park SH, Lee KW, Oh TJ, Misch CE, Shotwell J, Wang HL. Effect of absorbable membranes on sandwich bone augmentation. Clin Oral Implants Res. 2008; 19:32–41.
Article37. Weng D, Hürzeler MB, Quiñones CR, Ohlms A, Caffesse RG. Contribution of the periosteum to bone formation in guided bone regeneration. A study in monkeys. Clin Oral Implants Res. 2000; 11:546–554.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- Histomorphometric evaluation of bone healing with fully interconnected microporous biphasic calcium phosphate ceramics in rabbit calvarial defects
- The biological effect of cyanoacrylate-combined calcium phosphate in rabbit calvarial defects
- The effects of bone regeneration in rabbit calvarial defect with particulated and block type of hydroxyapatite
- Bone regeneration capacity of two different macroporous biphasic calcium materials in rabbit calvarial defect