J Korean Acad Periodontol.
2009 Aug;39(Suppl):223-230.
Bone regeneration capacity of two different macroporous biphasic calcium materials in rabbit calvarial defect
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, College of Dentistry, Yonsei University, Korea. shchoi726@yuhs.ac
- 2Department of Dentistry, College of Medicine, Kwandong University, Myongji Hospital, Korea.
Abstract
- ABSTRACT
PURPOSE: Synthetic bone products such as biphasic calcium phosphate (BCP) are mixtures of hydroxyapatite (HA) and a- tricalcium phosphate (a- TCP). In periodontal therapies and implant treatments, BCP provides to be a good bone reconstructive material since it has a similar chemical composition to biological bone apatites. The purpose of this study was to compare bone regeneration capacity of two commercially available BCP.
METHODS
Calvarial defects were prepared in sixteen 9-20 months old New Zealand White male rabbits. BCP with HA and a- TCP (70:30) and BCP with Silicon-substituted hydroxyapatite (Si-HA) and a-TCP (60:40) particles were filled in each defect. Control defects were filled with only blood clots. Animals were sacrificed at 4 and 8 week postoperatively. Histomorphometric analysis was performed.
RESULTS
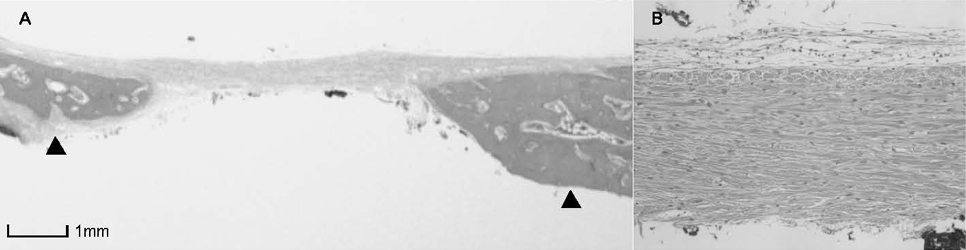
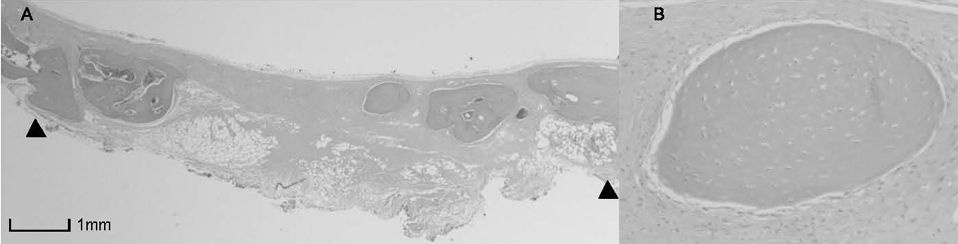
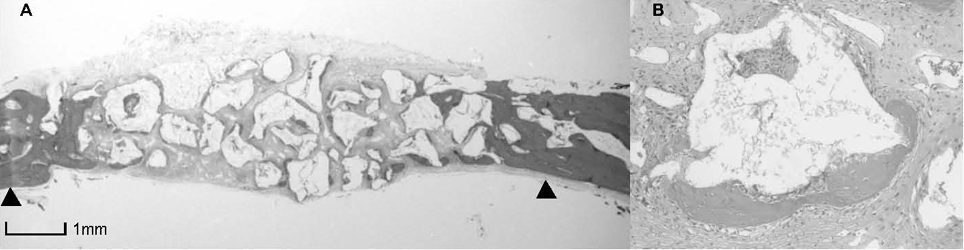
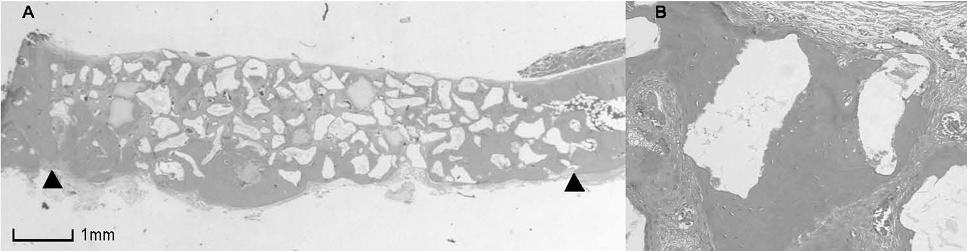
BCP with HAand a- TCP 8 weeks group and BCP with Si-HA and a- TCP 4 and 8 weeks groups showed statistically significant in crease (P<0.05) in augmented area than control group. Newly formed bone area after 4 and 8 weeks was similar among all the groups. Residual materials were slightly more evident in BCP with HA and a- TCP 8 weeks group.
CONCLUSIONS
Based on histological results, BCP with HA and a- TCP and BCP with Si-HA and a- TCP appears to demonstrate acceptable space maintaining capacity and elicit significant new bone formation when compared to natural bone healing in 4 and 8 week periods.
MeSH Terms
Figure
Reference
-
1. Barboza EP. Clinical and histologic evaluation of the demineralized freeze-dried bone membrane used for ridge augmentation. Int J Periodontics Restorative Dent. 1999. 19:601–607.2. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003. 14:195–200.3. Daculsi G, Passuti N, Martin S, et al. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990. 24:379–396.
Article4. LeGeros RZ, Parsons JR, Daculsi G, et al. Significance of the porosity and physical chemistry of calcium phosphate ceramics. Biodegradation-bioresorption. Ann N Y Acad Sci. 1988. 523:268–271.
Article5. Levin MP, Getter L, Adrian J, Cutright DE. Healing of periodontal defects with ceramic implants. J Clin Periodontol. 1974. 1:197–205.
Article6. Levin MP, Getter L, Cutright DE, Bhaskar SN. Biodegradable ceramic in periodontal defects. Oral Surg Oral Med Oral Pathol. 1974. 38:344–351.
Article7. Yukna RA, Cassingham RJ, Caudill RF, et al. Six month evaluation of Calcitite (hydroxyapatite ceramic) in periodontal osseous defects. Int J Periodontics Restorative Dent. 1986. 6:34–45.8. Froum SJ, Kushner L, Scopp IW, Stahl SS. Human clinical and histologic responses to Durapatite implants in intraosseous lesions. Case reports. J Periodontol. 1982. 53:719–725.
Article9. Moskow BS, Lubarr A. Histological assessment of human periodontal defect after durapatite ceramic implant. Report of a case. J Periodontol. 1983. 54:455–462.
Article10. Ellinger RF, Nery EB, Lynch KL. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int J Periodontics Restorative Dent. 1986. 6:22–33.11. Karabuda C, Ozdemir O, Tosun T, Anil A, Olgac V. Histological and clinical evaluation of 3 different grafting materials for sinus lifting procedure based on 8 cases. J Periodontol. 2001. 72:1436–1442.
Article12. Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981. 00:259–278.
Article13. Jarcho M. Biomaterial aspects of calcium phosphates. Properties and applications. Dent Clin North Am. 1986. 30:25–47.14. Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992. 63:729–735.
Article15. Block MS, Kent JN. A comparison of particulate and solid root forms of hydroxylapatite in dog extraction sites. J Oral Maxillofac Surg. 1986. 44:89–93.
Article16. Lee J, Jung U, Kim C, Choi S, Cho K. Maxillary sinus augmentation using macroporous biphasic calcium phosphate (MBCP™): Three case report with histologic evaluation. J Korean Acad Periodontol. 2006. 36:567–577.
Article17. Daculsi G, LeGeros RZ, NeryE , Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989. 23:883–894.
Article18. Bertoni E, Bigi A, Cojazzi G, et al. Nanocrystals of magnesium and fluoride substituted hydroxyapatite. J Inorg Biochem. 1998. 72:29–35.
Article19. Skrtic D, Antonucci JM, Eanes ED, Brunworth RT. Silicaand zirconia-hybridized amorphous calcium phosphate: effect on transformation to hydroxyapatite. J Biomed Mater Res. 2002. 59:597–604.
Article20. Hollinger JO, Kleinschmidt JC. The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg. 1990. 1:60–68.
Article21. Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:638–646.22. Sandy J, Davies M, Prime S, Farndale R. Signal pathways that transduce growth factor-stimulated mitogenesis in bone cells. Bone. 1998. 23:17–26.
Article23. Horner A, Bord S, Kemp P, Grainger D, Compston JE. Distribution of platelet-derived growth factor (PDGF) A chain mRNA, protein, and PDGF-alpha receptor in rapidly forming human bone. Bone. 1996. 19:353–362.
Article24. Lee YJ, Jung SW, Chae GJ, Cho KS, Kim CS. The effect of recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate block system on bone formation in rat calvarial defects. J Korean Acad Periodontol. 2007. 37:397–407.
Article25. Wikesjo UM, Huang YH, Polimeni G, Qahash M. Bone morphogenetic proteins: a realistic alternative to bone grafting for alveolar reconstruction. Oral Maxillofac Surg Clin North Am. 2007. 19:535–551. vi–vii.
Article26. Klein CP, Driessen AA, de Groot K, van den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mater Res. 1983. 17:769–784.
Article27. Kwon S, Jun Y, Hong S. Synthesis and dissolution behavior of β-TCP and HA/β-TCP composite powders. J Euro Ceram Soc. 2003. 23:1039–1045.
Article28. Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G. Macroporous biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998. 19:133–139.
Article29. LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988. 2:164–180.
Article30. Metsger DS, Driskell TD, Paulsrud JR. Tricalcium phosphate ceramic--a resorbable bone implant: review and current status. J Am Dent Assoc. 1982. 105:1035–1038.
Article31. Um YJ, Hong JY, Kim ST, et al. Bone formation of newly developed biphasic calcium phosphate in rabbit calvarial defect model : A pilot study. J Korean Acad Periodontol. 2008. 38:163–170.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- The biological effect of cyanoacrylate-combined calcium phosphate in rabbit calvarial defects
- The effect of micro-macroporous biphasic calcium phosphate incorporated with polyphosphate on exophytic bone regeneration
- Novel Calcium Phosphate Glass for Hard-Tissue Regeneration
- Effectiveness of biphasic calcium phosphate block bone substitutes processed using a modified extrusion method in rabbit calvarial defects