J Periodontal Implant Sci.
2013 Apr;43(2):64-71.
Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. jkchai@yuhs.ac
Abstract
- PURPOSE
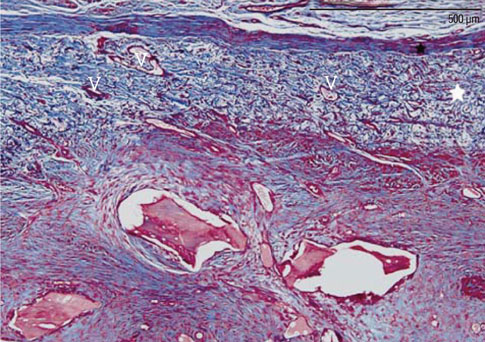
The objective of this study was to elucidate the role of collagen membranes (CMs) when used in conjunction with bovine hydroxyapatite particles incorporated with collagen matrix (BHC) for lateral onlay grafts in dogs.
METHODS
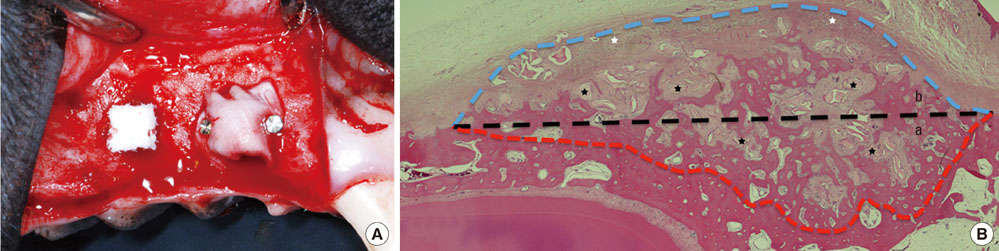
The first, second, and third premolars in the right maxilla of mongrel dogs (n=5) were extracted. After 2 months of healing, two BHC blocks (4 mmx4 mmx5 mm) were placed on the buccal ridge, one with and one without the coverage by a CM. The animals were sacrificed after 8 weeks for histometric analysis.
RESULTS
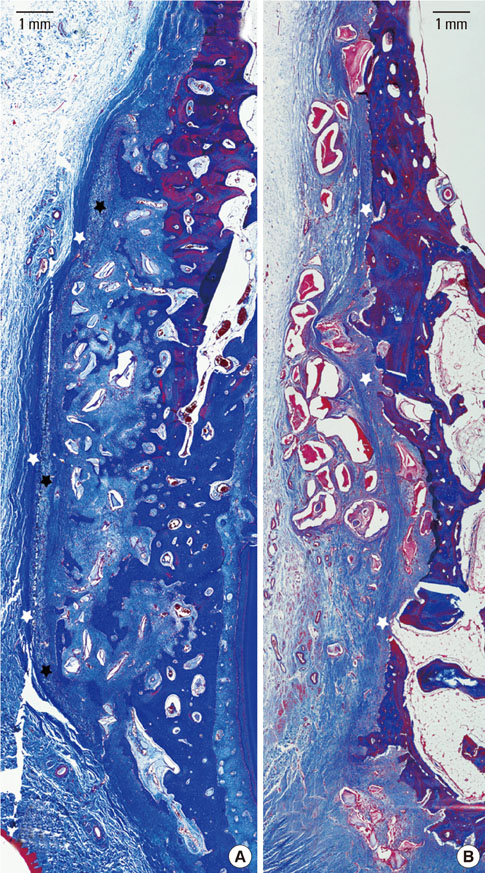
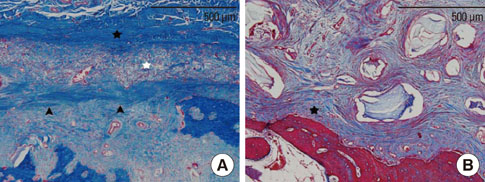
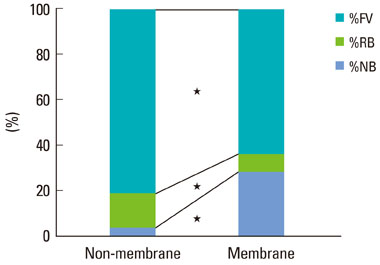
The collagen network of the membranes remained and served as a barrier. The quantity and quality of bone regeneration were all significantly greater in the membrane group than in the no-membrane group (P<0.05).
CONCLUSIONS
The use of barrier membranes in lateral onlay grafts leads to superior new bone formation and bone quality compared with bone graft alone.
Keyword
MeSH Terms
Figure
Reference
-
1. Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994. 9:13–29.2. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984. 11:494–503.
Article3. Buser D, Dula K, Belser U, Hirt HP, Berthold H. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993. 13:29–45.4. Fugazzotto PA. Maintenance of soft tissue closure following guided bone regeneration: technical considerations and report of 723 cases. J Periodontol. 1999. 70:1085–1097.
Article5. Rintala AE, Ranta R. Periosteal flaps and grafts in primary cleft repair: a follow-up study. Plast Reconstr Surg. 1989. 83:17–24.6. Roberts SJ, Geris L, Kerckhofs G, Desmet E, Schrooten J, Luyten FP. The combined bone forming capacity of human periosteal derived cells and calcium phosphates. Biomaterials. 2011. 32:4393–4405.
Article7. Eyre-Brook AL. The periosteum: its function reassessed. Clin Orthop Relat Res. 1984. 189:300–307.8. Simion M, Nevins M, Rocchietta I, Fontana F, Maschera E, Schupbach P, et al. Vertical ridge augmentation using an equine block infused with recombinant human platelet-derived growth factor-BB: a histologic study in a canine model. Int J Periodontics Restorative Dent. 2009. 29:245–255.9. Simion M, Rocchietta I, Dellavia C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: report of two cases. Int J Periodontics Restorative Dent. 2007. 27:109–115.10. Hammerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res. 2008. 19:19–25.
Article11. Zecha PJ, Schortinghuis J, van der Wal JE, Nagursky H, van den Broek KC, Sauerbier S, et al. Applicability of equine hydroxyapatite collagen (eHAC) bone blocks for lateral augmentation of the alveolar crest. A histological and histomorphometric analysis in rats. Int J Oral Maxillofac Surg. 2011. 40:533–542.
Article12. Araujo M, Linder E, Wennstrom J, Lindhe J. The influence of Bio-Oss Collagen on healing of an extraction socket: an experimental study in the dog. Int J Periodontics Restorative Dent. 2008. 28:123–135.13. Heberer S, Al-Chawaf B, Hildebrand D, Nelson JJ, Nelson K. Histomorphometric analysis of extraction sockets augmented with Bio-Oss Collagen after a 6-week healing period: a prospective study. Clin Oral Implants Res. 2008. 19:1219–1225.
Article14. Tawil G, Mawla M. Sinus floor elevation using a bovine bone mineral (Bio-Oss) with or without the concomitant use of a bilayered collagen barrier (Bio-Gide): a clinical report of immediate and delayed implant placement. Int J Oral Maxillofac Implants. 2001. 16:713–721.15. Yamada S, Shima N, Kitamura H, Sugito H. Effect of porous xenographic bone graft with collagen barrier membrane on periodontal regeneration. Int J Periodontics Restorative Dent. 2002. 22:389–397.16. Donos N, Lang NP, Karoussis IK, Bosshardt D, Tonetti M, Kostopoulos L. Effect of GBR in combination with deproteinized bovine bone mineral and/or enamel matrix proteins on the healing of critical-size defects. Clin Oral Implants Res. 2004. 15:101–111.
Article17. Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjo UM, et al. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004. 75:229–235.
Article18. Brunel G, Benque E, Elharar F, Sansac C, Duffort JF, Barthet P, et al. Guided bone regeneration for immediate non-submerged implant placement using bioabsorbable materials in Beagle dogs. Clin Oral Implants Res. 1998. 9:303–312.
Article19. Sandberg E, Dahlin C, Linde A. Bone regeneration by the osteopromotion technique using bioabsorbable membranes: an experimental study in rats. J Oral Maxillofac Surg. 1993. 51:1106–1114.
Article20. Zellin G, Gritli-Linde A, Linde A. Healing of mandibular defects with different biodegradable and non-biodegradable membranes: an experimental study in rats. Biomaterials. 1995. 16:601–609.
Article21. Tawil G, El-Ghoule G, Mawla M. Clinical evaluation of a bilayered collagen membrane (Bio-Gide) supported by autografts in the treatment of bone defects around implants. Int J Oral Maxillofac Implants. 2001. 16:857–863.22. Blumenthal NM. A clinical comparison of collagen membranes with e-PTFE membranes in the treatment of human mandibular buccal class II furcation defects. J Periodontol. 1993. 64:925–933.
Article23. Pitaru S, Tal H, Soldinger M, Azar-Avidan O, Noff M. Collagen membranes prevent the apical migration of epithelium during periodontal wound healing. J Periodontal Res. 1987. 22:331–333.
Article24. Pitaru S, Tal H, Soldinger M, Noff M. Collagen membranes prevent apical migration of epithelium and support new connective tissue attachment during periodontal wound healing in dogs. J Periodontal Res. 1989. 24:247–253.
Article25. Stavropoulos A, Wikesjo UM. Influence of defect dimensions on periodontal wound healing/regeneration in intrabony defects following implantation of a bovine bone biomaterial and provisions for guided tissue regeneration: an experimental study in the dog. J Clin Periodontol. 2010. 37:534–543.
Article26. Tadjoedin ES, de Lange GL, Bronckers AL, Lyaruu DM, Burger EH. Deproteinized cancellous bovine bone (Bio-Oss) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. J Clin Periodontol. 2003. 30:261–270.
Article27. Agis H, Magdalenko M, Stogerer K, Watzek G, Gruber R. Collagen barrier membranes decrease osteoclastogenesis in murine bone marrow cultures. Clin Oral Implants Res. 2010. 21:656–661.
Article28. Bassil J, Senni K, Changotade S, Baroukh B, Kassis C, Naaman N, et al. Expression of MMP-2, 9 and 13 in newly formed bone after sinus augmentation using inorganic bovine bone in human. J Periodontal Res. 2011. 46:756–762.
Article29. Araujo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clin Oral Implants Res. 2010. 21:55–64.
Article30. Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997. 8:117–124.
Article31. Jung UW, Lee JS, Park WY, Cha JK, Hwang JW, Park JC, et al. Periodontal regenerative effect of a bovine hydroxyapatite/collagen block in one-wall intrabony defects in dogs: a histometric analysis. J Periodontal Implant Sci. 2011. 41:285–292.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Behavior of Fibroblasts on a Porous Hyaluronic Acid Incorporated Collagen Matrix
- The Durability of Elastin-Incorporated Collagen Matrix for Dermal Substitute in Vitro Condition
- Fibronectin, Type IV Collagen, and Concanavalin A in Human Bruch's Membrane
- Periodontal regenerative effect of a bovine hydroxyapatite/collagen block in one-wall intrabony defects in dogs: a histometric analysis
- Collagen Matrix Contraction by Cell of Proliferative Vitreoretinopathy