J Periodontal Implant Sci.
2011 Apr;41(2):54-59.
Identification of mono- or poly-specific monoclonal antibody to Porphyromonas gingivalis heat-shock protein 60
- Affiliations
-
- 1Department of Periodontology, Pusan National University School of Dentistry, Yangsan, Korea. jrapa@pusan.ac.kr
- 2Department of Biochemistry, Pusan National University School of Medicine, Yangsan, Korea.
- 3Department of Pharmacology, Pusan National University School of Medicine, Yangsan, Korea.
- 4Department of Microbiology and Immunology, Seoul National University School of Dentistry, Seoul, Korea.
Abstract
- PURPOSE
The aim of this study was to define the immunoreactive specificity of Porphyromonas gingivalis (P. gingivalis) heat shock protein (HSP) 60 in periodontitis and atherosclerosis.
METHODS
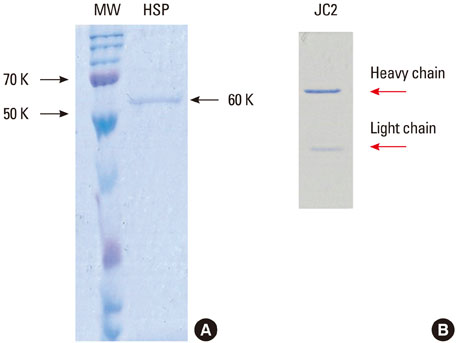
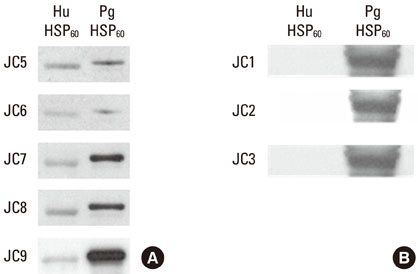
In an attempt to define the cross-reactive bacterial heat-shock protein with human self-antigen at molecular level, we have introduced a novel strategy for cloning hybridoma producing anti-P. gingivalis HSP 60 which is polyreactive to bacterial HSPs or to the human homolog.
RESULTS
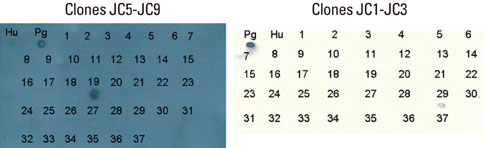
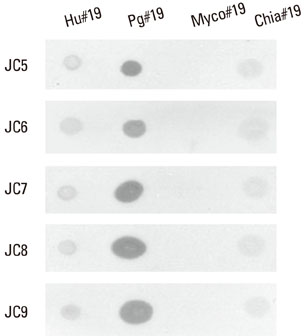
Five cross-reactive clones were obtained which recognized the #19 peptide (TLVVNRLRGSLKICAVKAPG) among 37 synthetic peptides (20-mer, 5 amino acids overlapping) spanning the whole molecule of P. gingivalis HSP 60. We have also established three anti-P. gingivalis HSP 60 monoclonal antibodies demonstrating mono-specificity. These clones recognized the #29 peptide (TVPGGGTTYIRAIAALEGLK).
CONCLUSIONS
Peptide #19 and #29 of P. gingivalis HSP 60 might be important immunoreactive epitopes in the immunopathogenic mechanism of bacterial antigen-triggered autoimmune diseases.
Keyword
MeSH Terms
-
Amino Acids
Antibodies
Antibodies, Monoclonal
Autoimmune Diseases
Chaperonin 60
Clone Cells
Cloning, Organism
Epitopes
Heat-Shock Proteins
Humans
Hybridomas
Peptides
Periodontitis
Porphyromonas
Porphyromonas gingivalis
Sensitivity and Specificity
Amino Acids
Antibodies
Antibodies, Monoclonal
Chaperonin 60
Epitopes
Heat-Shock Proteins
Peptides
Figure
Reference
-
1. Choi JI, Chung SW, Kang HS, Rhim BY, Kim SJ, Kim SJ. Establishment of Porphyromonas gingivalis heat-shock-protein-specific T-cell lines from atherosclerosis patients. J Dent Res. 2002. 81:344–348.
Article2. Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, et al. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004. 83:936–940.
Article3. Choi J, Chung SW, Kim SJ, Kim SJ. Establishment of Porphyromonas gingivalis-specific T-cell lines from atherosclerosis patients. Oral Microbiol Immunol. 2001. 16:316–318.
Article4. Choi JI, Kang HS, Park YM, Kim SJ, Kim US. Identification of T-cell epitopes of Porphyromonas gingivalis heat-shock-protein 60 in periodontitis. Oral Microbiol Immunol. 2004. 19:1–5.
Article5. Lee JY, Yi NN, Kim US, Choi JS, Kim SJ, Choi JI. Porphyromonas gingivalis heat shock protein vaccine reduces the alveolar bone loss induced by multiple periodontopathogenic bacteria. J Periodontal Res. 2006. 41:10–14.
Article6. Choi JI, Choi KS, Yi NN, Kim US, Choi JS, Kim SJ. Recognition and phagocytosis of multiple periodontopathogenic bacteria by anti-Porphyromonas gingivalis heat-shock protein 60 antisera. Oral Microbiol Immunol. 2005. 20:51–55.
Article7. Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005. 6:353–360.
Article8. Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007. 1113:217–237.
Article9. Lee J, Suh J, Choi J. B-1 cell-derived monoclonal antibodies and costimulatory molecules. J Surg Res. 2009. 154:293–298.
Article10. Notkins AL. Polyreactive antibodies and polyreactive antigen-binding B (PAB) Cells. Curr Top Microbiol Immunol. 2000. 252:241–249.
Article11. Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007. 29:219–228.
Article12. Choi JI, Borrello MA, Cutler CW, Zauderer M. Prior Immunization with fusobacterium nucleatum interferes with opsonophagocytosis function of sera against Porphyromonas gingivalis. J Korean Acad Periodontol. 2000. 30:105–110.
Article13. Choi JI, Borrello MA, Smith ES, Zauderer M. Polarization of Porphyromonas gingivalis-specific helper T-cell subsets by prior immunization with Fusobacterium nucleatum. Oral Microbiol Immunol. 2000. 15:181–187.
Article14. Choi J, Borrello MA, Smith E, Cutler CW, Sojar H, Zauderer M. Prior exposure of mice to Fusobacterium nucleatum modulates host response to Porphyromonas gingivalis. Oral Microbiol Immunol. 2001. 16:338–344.
Article15. Shinnick TM. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991. 167:145–160.
Article16. Maeda H, Miyamoto M, Kokeguchi S, Kono T, Nishimura F, Takashiba S, et al. Epitope mapping of heat shock protein 60 (GroEL) from Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2000. 28:219–224.
Article17. Bak J, Kim SJ, Choi J. Epitope specificity of Porphyromonas gingivalis heat shock protein for T-cell and/or B-cell in human atherosclerosis. J Korean Acad Periodontol. 2003. 33:179–191.
Article18. van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005. 5:318–330.
Article19. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006. 6:508–519.
Article20. Wick G, Perschinka H, Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001. 22:665–669.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibody response of periodontal patients to Porphyromonas gingivalis heat shock protein
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Development of monoclonal antibody against Porphyromonas gingivalis heat shock protein
- Epitope specificity of Porphyromonas gingivalis heat shock protein for T-cell and/or B-cell in human atherosclerosis
- T-and cross-reactive B-cell epitopes of Porphyromonas gingivalis and human heat shock protein 60 in atherosclerosis