J Gastric Cancer.
2016 Jun;16(2):115-119. 10.5230/jgc.2016.16.2.115.
A Case of Long-Term Complete Remission of Advanced Gastric Adenocarcinoma with Liver Metastasis
- Affiliations
-
- 1Department of Internal Medicine, Eulji University Hospital, Daejeon, Korea. naeyu46@eulji.ac.kr
- 2Department of Pathology, Eulji University Hospital, Daejeon, Korea.
- 3Department of Emergency Medicine, Eulji University Hospital, Daejeon, Korea.
- 4Department of Radiology, Eulji University Hospital, Daejeon, Korea.
- KMID: 2328079
- DOI: http://doi.org/10.5230/jgc.2016.16.2.115
Abstract
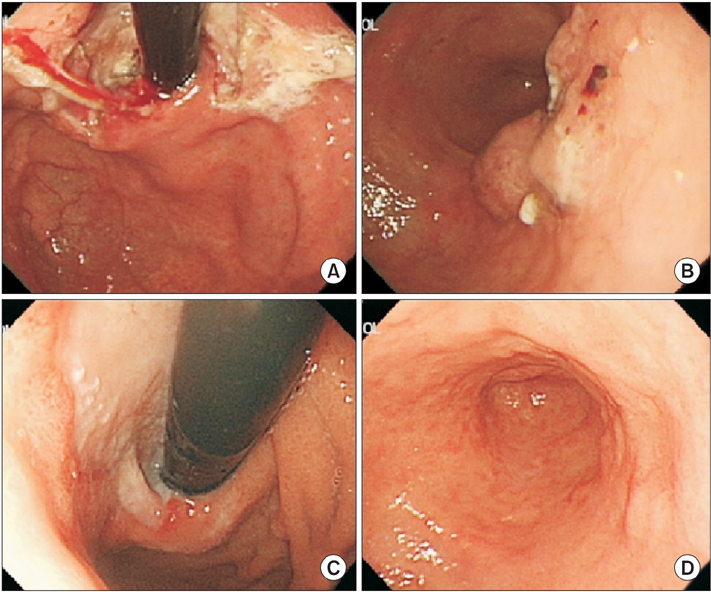
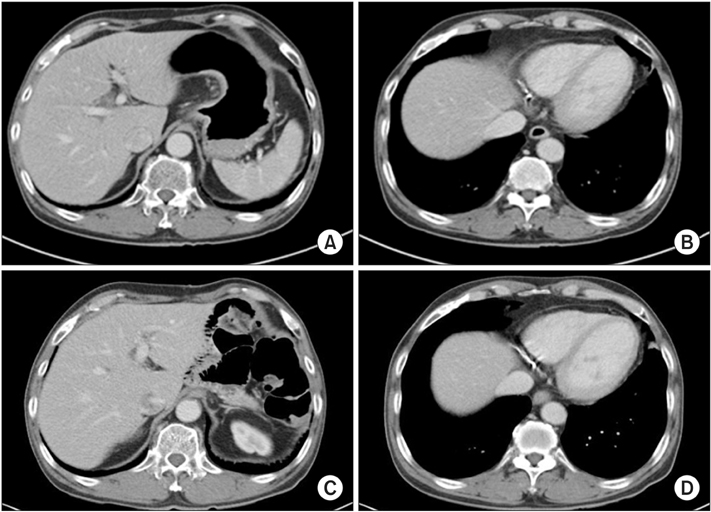
- We report the case of a patient with gastric adenocarcinoma with multiple liver metastases. This patient showed complete remission for more than 68 months after S-1/cisplatin combination chemotherapy and radical total gastrectomy. The patient, a 63-year-old man, presented with dyspepsia and difficulty in swallowing. Endoscopic findings showed a huge ulcero-infiltrative mass at the lesser curvature of the mid-body, extending to the distal esophagus. Biopsy revealed a poorly differentiated tubular adenocarcinoma. An abdominal computed tomography scan demonstrated multiple hepatic metastases. S-1/cisplatin combination chemotherapy was initiated, and following completion of six cycles of chemotherapy, the gastric masses and hepatic metastatic lesions had disappeared on abdominal computed tomography. Radical total gastrectomy and D2 lymphadenectomy combined with splenectomy were performed. The patient underwent three cycles of S-1/cisplatin combination chemotherapy followed by tegafur-uracil therapy for 1 year. He remained in complete remission for more than 68 months after surgery.
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.2. Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168.3. Rosati G, Ferrara D, Manzione L. New perspectives in the treatment of advanced or metastatic gastric cancer. World J Gastroenterol. 2009; 15:2689–2692.4. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9:215–221.5. Iwahashi M, Nakamori M, Tani M, Yamaue H, Sakaguchi S, Nakamura M, et al. Complete response of highly advanced gastric cancer with peritoneal dissemination after new combined chemotherapy of S-1 and low-dose cisplatin: report of a case. Oncology. 2001; 61:16–22.6. Mitomi H, Kishimoto I, Amemiya A, Kaneda G, Adachi K, Shimoda T, et al. Advanced gastric cancer showing long-term complete remission in response to S-1 monotherapy: two case reports. Cases J. 2008; 1:405.7. Suzuki Y, Kawasaki N, Ishibashi Y, Yanaga K, Kashiwagi H, Koba K, et al. A case of stage IV gastric cancer: long term remmision achieved with S-1 mono chemotherapy. JMAJ. 2006; 49:219–223.8. Kochi M, Fujii M, Kanamori N, Kaiga T, Takahashi T, Kobayashi M, et al. Neoadjuvant chemotherapy with S-1 and CDDP in advanced gastric cancer. J Cancer Res Clin Oncol. 2006; 132:781–785.9. Satoh S, Hasegawa S, Ozaki N, Okabe H, Watanabe G, Nagayama S, et al. Retrospective analysis of 45 consecutive patients with advanced gastric cancer treated with neoadjuvant chemotherapy using an S-1/CDDP combination. Gastric Cancer. 2006; 9:129–135.10. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820.11. Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiguchi N, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014; 15:886–893.12. Yoon SY, Kim SY, Cho YH, Chung HW, So Y, Lee HM. Hepatic metastases of gastric adenocarcinoma showing metabolic remission on FDG-PET despite an increase in size on CT. Cancer Res Treat. 2009; 41:100–103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hepatoid Adenocarcinoma without Involving Liver Parenchyme
- Early Gastric Mucosal Cancer Associated with Synchronous Liver Metastasis
- Minimal Change Disease Associated with Gastric Adenocarcinoma and Multiple Liver Metastasis
- Complete Remission after Chemotherapy in Stage IV Gastric Cancer
- Hepatoid Adenocarcinoma of the Stomach with Liver Metastasis