J Korean Soc Radiol.
2013 May;68(5):375-383.
Magnetic Resonance Imaging Features of Neuromyelitis Optica
- Affiliations
-
- 1Department of Radiology, Chungnam National University College of Medicine, Chungnam National University Hospital, Daejeon, Korea. cjsong@cnu.ac.kr
- 2Department of Neurology, Chungnam National University College of Medicine, Chungnam National University Hospital, Daejeon, Korea.
Abstract
- PURPOSE
To report the magnetic resonance (MR) imaging features of the spinal cord and brain in patients of neuromyelitis optica (NMO).
MATERIALS AND METHODS
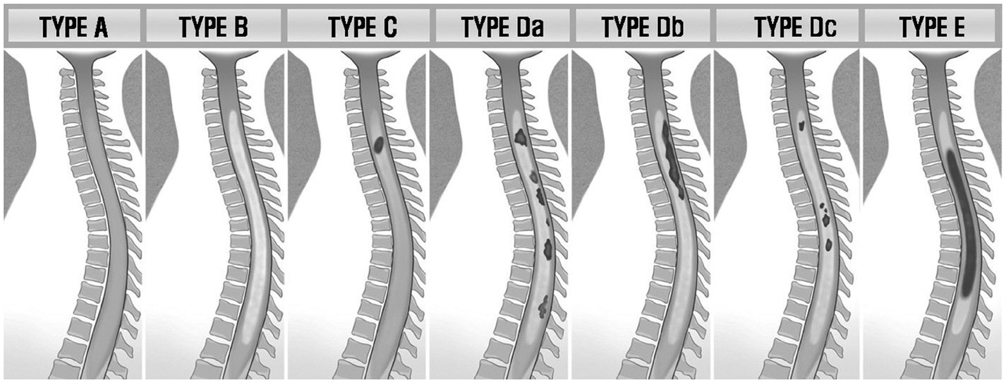
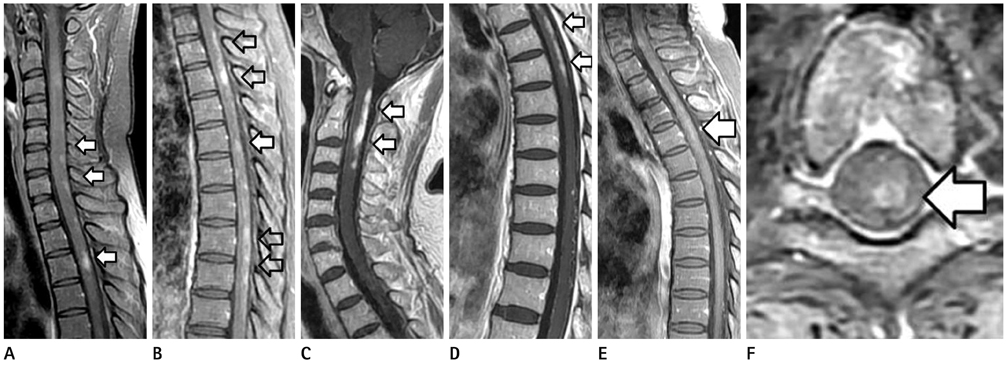
Between January 2001 and March 2010, the MR images (spinal cord, brain, and orbit) and the clinical and serologic findings of 11 NMO patients were retrospectively reviewed. The contrast-enhancement of the spinal cord was performed (20/23). The presence and pattern of the contrast-enhancement in the spinal cord were classified into 5 types.
RESULTS
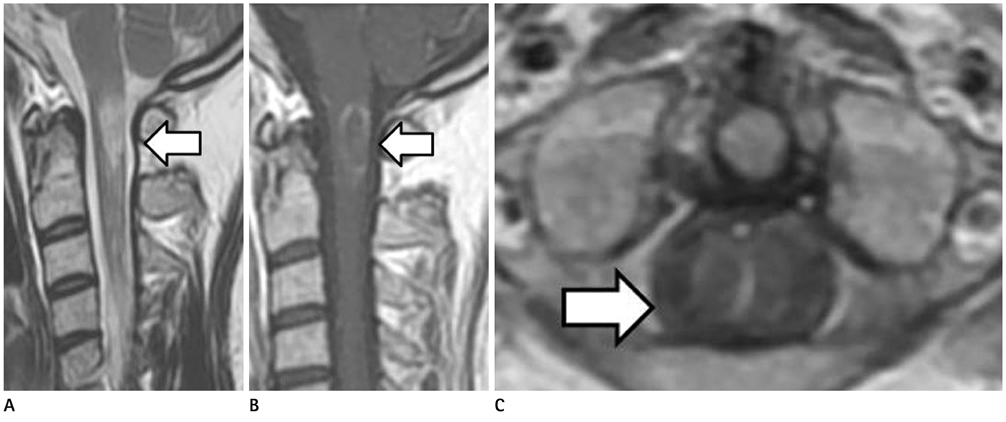
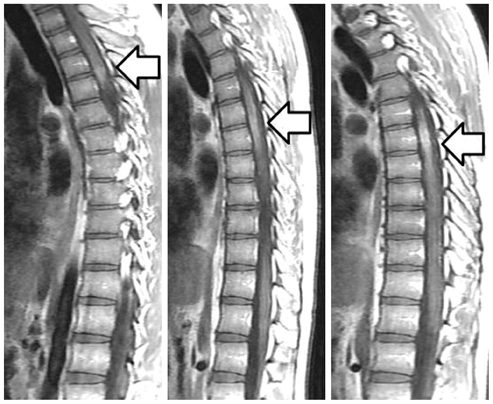
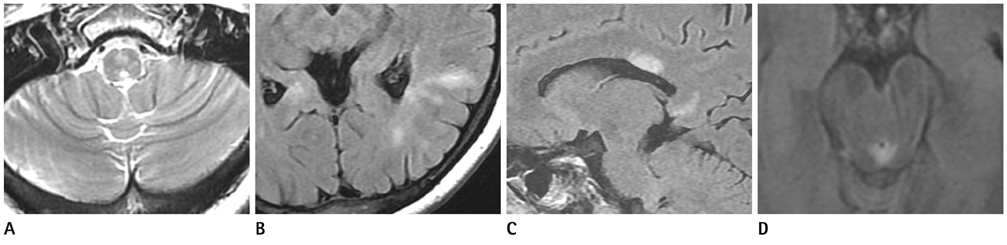
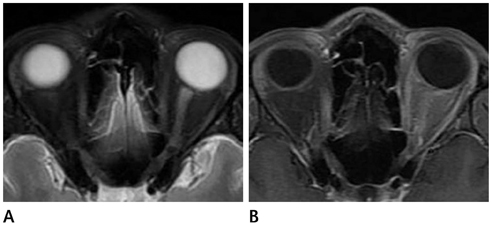
Acute myelitis was monophasic in 8 patients (8/11, 72.7%); and optic neuritis preceded acute myelitis in most patients. Longitudinally extensive cord lesion (average, 7.3 vertebral segments) was involved. The most common type was the diffuse and subtle enhancement of the spinal cord with a multifocal nodular, linear or segmental intense enhancement (45%). Most of the brain lesions (5/11, 10 lesions) were located in the brain stem, thalamus and callososeptal interphase. Anti-Ro autoantibody was positive in 2 patients, and they showed a high relapse rate of acute myelitis. Anti-NMO IgG was positive in 4 patients (4/7, 66.7%).
CONCLUSION
The imaging findings of acute myelitis in NMO may helpful in making an early diagnosis of NMO which can result in a severe damage to the spinal cord, and to make a differential diagnosis of multiple sclerosis and inflammatory diseases of the spinal cord such as toxocariasis.
MeSH Terms
-
Brain
Brain Stem
Diagnosis, Differential
Early Diagnosis
Humans
Immunoglobulin G
Interphase
Magnetic Resonance Imaging
Magnetic Resonance Spectroscopy
Magnetics
Magnets
Multiple Sclerosis
Myelitis
Neuromyelitis Optica
Optic Neuritis
Recurrence
Retrospective Studies
Sjogren's Syndrome
Spinal Cord
Spine
Thalamus
Toxocariasis
Immunoglobulin G
Figure
Reference
-
1. Devic E. Myélite subaiguë compliquée de névrite optique. Bull Med. 1894. 8:1033–1103.2. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999. 53:1107–1114.3. Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002. 125(Pt 7):1450–1461.4. Galetta SL, Bennett J. Neuromyelitis optica is a variant of multiple sclerosis. Arch Neurol. 2007. 64:901–903.5. Weinshenker BG. Neuromyelitis optica is distinct from multiple sclerosis. Arch Neurol. 2007. 64:899–901.6. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004. 364:2106–2112.7. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005. 202:473–477.8. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006. 66:1485–1489.9. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002. 61:554–558.10. Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003. 2:117–127.11. Nakashima I, Fujihara K, Miyazawa I, Misu T, Narikawa K, Nakamura M, et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgG. J Neurol Neurosurg Psychiatry. 2006. 77:1073–1075.12. Tanaka K, Tani T, Tanaka M, Saida T, Idezuka J, Yamazaki M, et al. Anti-aquaporin 4 antibody in selected Japanese multiple sclerosis patients with long spinal cord lesions. Mult Scler. 2007. 13:850–855.13. Chong H, Kermode A, Tan C. The role of anti-aquaporin-4 antibody in asian patients with multiple sclerosis: confusions and controversies. Neurol Asia. 2007. 12:135–139.14. Matsuoka T, Matsushita T, Kawano Y, Osoegawa M, Ochi H, Ishizu T, et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain. 2007. 130(Pt 5):1206–1223.15. Park KH, Kim SW, Kim SK. Multiple sclerosis in Busan Korea clinical features and prevalence. J Korean Neurol Assoc. 1983. 1:29–36.16. Lee SS, Sohn EH, Nam SW. Preliminary studies on the clinical features of multiple sclerosis in Korea. J Clin Neurol. 2006. 2:231–237.17. Cho YJ, Jeon BS, Kim YH, Chang KH. Clinical features and outcomes from diagnostic work-up in definite multiple sclerosis. J Korean Neurol Assoc. 1999. 17:823–828.18. O'Riordan JI, Gallagher HL, Thompson AJ, Howard RS, Kingsley DP, Thompson EJ, et al. Clinical, CSF, and MRI findings in Devic's neuromyelitis optica. J Neurol Neurosurg Psychiatry. 1996. 60:382–387.19. Lalan S, Khan M, Schlakman B, Penman A, Gatlin J, Herndon R. Differentiation of neuromyelitis optica from multiple sclerosis on spinal magnetic resonance imaging. Int J MS Care. 2012. 14:209–214.20. Lee IH, Kim ST, Oh DK, Kim HJ, Kim KH, Jeon P, et al. MRI findings of spinal visceral larva migrans of Toxocara canis. Eur J Radiol. 2010. 75:236–240.21. Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006. 63:390–396.22. Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006. 63:964–968.23. Hummers LK, Krishnan C, Casciola-Rosen L, Rosen A, Morris S, Mahoney JA, et al. Recurrent transverse myelitis associates with anti-Ro (SSA) autoantibodies. Neurology. 2004. 62:147–149.24. Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008. 65:78–83.25. Koo YS, Yoo JK, Kwon DY, Park MH, Koh SB, Kim BJ, et al. Neuromyelitis optica with positive anti-Ro and anti-La antibodies. J Korean Neurol Assoc. 2009. 27:446–448.26. Cho JH, Kim SM, Kim JH, Chu CK, Lee MH, Shin HW, et al. Two cases of primary sjogren's syndrome presenting as relapsing-remitting multiple sclerosis. J Korean Neurol Assoc. 2004. 22:410–413.27. Min JH, Kim HJ, Kim BJ, Lee KW, Sunwoo IN, Kim SM, et al. Brain abnormalities in Sjogren syndrome with recurrent CNS manifestations: association with neuromyelitis optica. Mult Scler. 2009. 15:1069–1076.28. Kim SM, Waters P, Vincent A, Kim SY, Kim HJ, Hong YH, et al. Sjogren's syndrome myelopathy: spinal cord involvement in Sjogren's syndrome might be a manifestation of neuromyelitis optica. Mult Scler. 2009. 15:1062–1068.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Neuromyelitis Optica
- A Case of Neuromyelitis Optica in Children
- Differential Diagnosis between Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder
- Late-onset Seronegative Neuromyelitis Optica Spectrum Disorder Mimicking Wernicke Encephalopathy
- A Case of Neuromyelitis Opitica (Devic Disease)