World J Mens Health.
2013 Aug;31(2):141-149.
Effects of Sesame Oil on the Reproductive Parameters of Diabetes Mellitus-Induced Male Rats
- Affiliations
-
- 1Department of Basic Sciences, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
- 2Department of Clinical Sciences, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran. fabrtir@yahoo.com
Abstract
- PURPOSE
The purpose of the present study was to investigate the effect of sesame oil on the reproductive parameters of diabetic male Wistar rats.
MATERIALS AND METHODS
The adult male rats in a split plot design were divided into normal (n=10), normal 5% (n=5; 5% sesame oil enriched diet), diabetic (Streptozocin induced diabetes; n=9), diabetic 5% (n=9; 5% sesame oil enriched diet), and diabetic 10% (n=9; 10% sesame oil enriched diet) groups. Diet supplementation continued for 56 days.
RESULTS
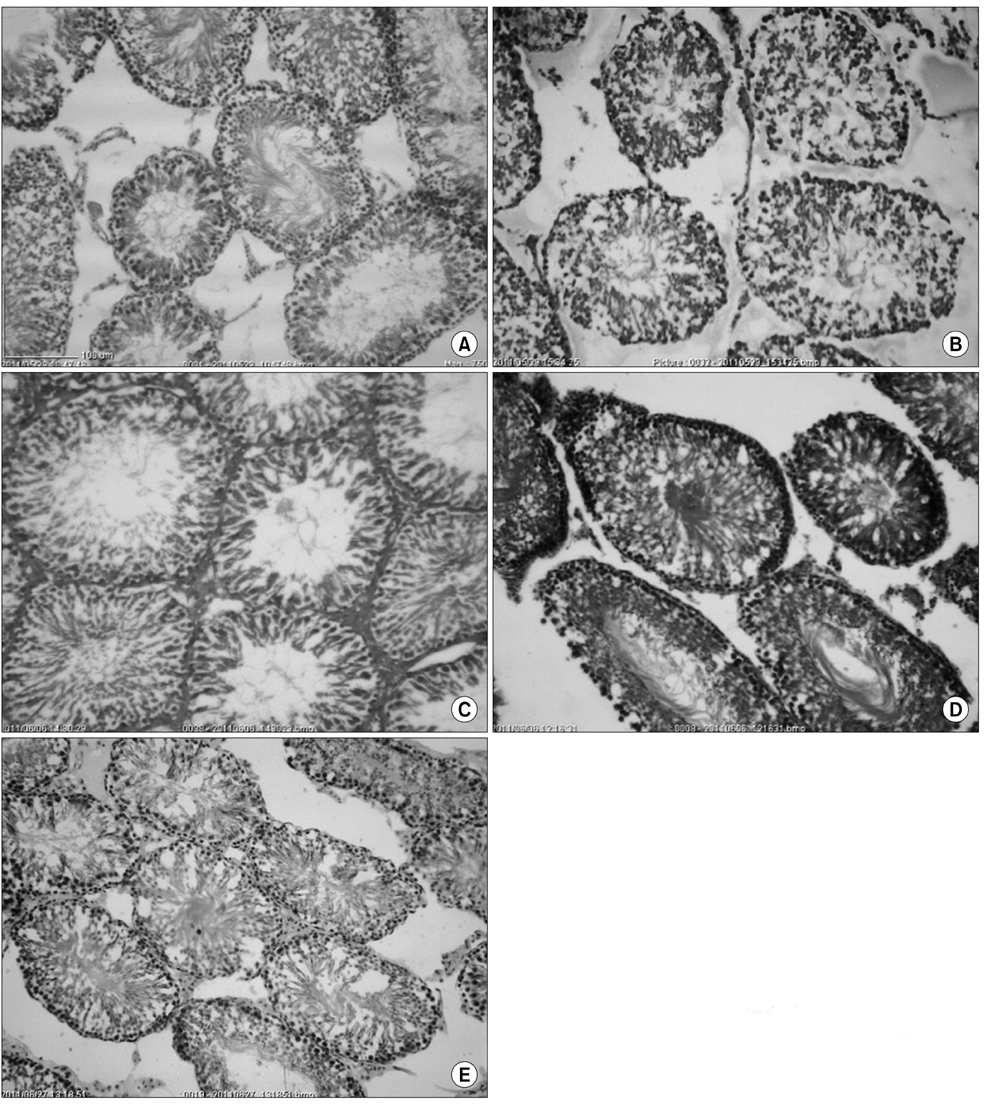
Sesame oil supplementation did not reduce the plasma glucose concentration of rats in the diabetic groups (p>0.05). The total spermatogonia, spermatocytes, Leydig cells/tubule, and the germ cell to Sertoli cell ratio were lower in the diabetic rats than the normal ones (p<0.05), and with the exception of spermatogonia counts, these values improved by the addition of sesame oil to the diet (p<0.05). The sperm progressive motility and viability were lower in the diabetic rats (p<0.05) and sesame oil supplementation did not improve them. Incorporation of sesame oil into the diet improved the plasma testosterone concentration of the diabetic rats in a dose-dependent manner (p<0.05).
CONCLUSIONS
In summary, sesame oil supplementation improved the reproductive parameters of diabetic rats at the levels of the testicular microstructure and function, but was not effective in protecting the epididymal sperm.
Keyword
MeSH Terms
Figure
Reference
-
1. Baccetti B, La Marca A, Piomboni P, Capitani S, Bruni E, Petraglia F, et al. Insulin-dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod. 2002; 17:2673–2677.
Article2. García-Díez LC, Corrales Hernandez JJ, Hernandez-Diaz J, Pedraz MJ, Miralles JM. Semen characteristics and diabetes mellitus: significance of insulin in male infertility. Arch Androl. 1991; 26:119–128.3. Mallidis C, Agbaje IM, Rogers DA, Glenn JV, Pringle R, Atkinson AB, et al. Advanced glycation end products accumulate in the reproductive tract of men with diabetes. Int J Androl. 2009; 32:295–305.
Article4. Navarro-Casado L, Juncos-Tobarra MA, Cháfer-Rudilla M, de Onzoño LÍ, Blázquez-Cabrera JA, Miralles-García JM. Effect of experimental diabetes and STZ on male fertility capacity. Study in rats. J Androl. 2010; 31:584–592.
Article5. Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 2006; 29:482–488.
Article6. Vignon F, Le Faou A, Montagnon D, Pradignac A, Cranz C, Winiszewsky P, et al. Comparative study of semen in diabetic and healthy men. Diabete Metab. 1991; 17:350–354.7. Gilja I, Parazajder J, Radej M, Kovacic M, Reljanovic M. Diabetes and retrograde ejaculation: possibilities of conservative treatment. Diabetologia Croatica. 1996; 25:83–86.8. Arikawe AP, Daramola AO, Odofin AO, Obika LF. Alloxan-induced and insulin-resistant diabetes mellitus affect semen parameters and impair spermatogenesis in male rats. Afr J Reprod Health. 2006; 10:106–113.9. Noguchi S, Ohba Y, Oka T. Involvement of epidermal growth factor deficiency in pathogenesis of oligozoospermia in streptozotocin-induced diabetic mice. Endocrinology. 1990; 127:2136–2140.
Article10. Komaki K, Ohno Y, Aoki N. Gonadal hormones and gonadal function in type 2 diabetes model OLETF (Otsuka Long Evans Tokushima Fatty) rats. Endocr J. 2005; 52:345–351.
Article11. Ali ST, Shaikh RN, Ashfaqsiddiqi N, Siddiqi PQ. Serum and urinary levels of pituitary--gonadal hormones in insulin-dependent and non-insulin-dependent diabetic males with and without neuropathy. Arch Androl. 1993; 30:117–123.
Article12. Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res Clin Pract. 2011; 91:61–66.
Article13. Giroix MH, Louchami K, Carpentier YA, Sener A, Malaisse WJ. Fatty acid pattern of pancreatic islet lipids in Goto-Kakizaki rats. Endocrine. 2010; 37:173–179.
Article14. Shrilatha B. Muralidhara. Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the Streptozotocin-diabetic rat. Int J Androl. 2007; 30:508–518.
Article15. Sawiress FA, Ziada MS, Bebawy WS, Amer HA. Effect of ginseng extract supplementation on testicular functions in diabetic rats. Endocr Regul. 2011; 45:139–148.
Article16. Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, et al. Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats. Toxicology. 2011; 282:69–81.
Article17. Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010; 48:2920–2924.
Article18. Rabbani SI, Devi K, Khanam S. Effect of rosiglitazone on the nicotinamide-streptozotocin induced type-2 diabetes mellitus mediated defects in sperm abnormalities and oxidative defense system in male Wistar rats. Acta Pharmaceutica Sciencia. 2010; 52:121–128.19. Rabbani SI, Devi K, Khanam S. Effect of metformin against the nicotinamide-streptozotocin induced sperm abnormalities in diabetic male wistar rats. Biomed Pharmacol J. 2009; 2:31–38.20. Soudamani S, Malini T, Balasubramanian K. Effects of streptozotocin-diabetes and insulin replacement on the epididymis of prepubertal rats: histological and histomorphometric studies. Endocr Res. 2005; 31:81–98.
Article21. Shittu Lukeman AJ, Shittu Remilekun K, Ogundife O, Tayo Adetokunbo O, Osunubi Abraham AA. Hypoglycaemia and improved testicular parameters in Sesamum radiatum treated normo-glycaemic adult male Sprague Dawley rats. Afr J Biotechnol. 2009; 8:2878–2886.22. Arumugam P, Ramesh S. Protective effects of sesame oil on 4-NQO-induced oxidative DNA damage and lipid peroxidation in rats. Drug Chem Toxicol. 2011; 34:116–119.
Article23. Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007; 47:651–673.
Article24. Umeda-Sawada R, Ogawa M, Igarashi O. The metabolism and n-6/n-3 ratio of essential fatty acids in rats: effect of dietary arachidonic acid and a mixture of sesame lignans (sesamin and episesamin). Lipids. 1998; 33:567–572.
Article25. Ide T, Lim JS, Odbayar TO, Nakashima Y. Comparative study of sesame lignans (sesamin, episesamin and sesamolin) affecting gene expression profile and fatty acid oxidation in rat liver. J Nutr Sci Vitaminol (Tokyo). 2009; 55:31–43.
Article26. WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva, Swittzerland: WHO press;2010. p. 22–44.27. McLachlan RI, Wreford NG, O'Donnell L, de Kretser DM, Robertson DM. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol. 1996; 148:1–9.
Article28. Van Beek ME, Meistrich ML. A method for quantifying synchrony in testes of rats treated with vitamin A deprivation and readministration. Biol Reprod. 1990; 42:424–431.29. Wu WH, Kang YP, Wang NH, Jou HJ, Wang TA. Sesame ingestion affects sex hormones, antioxidant status, and blood lipids in postmenopausal women. J Nutr. 2006; 136:1270–1275.
Article30. Hassan AA, Hassouna MM, Taketo T, Gagnon C, Elhilali MM. The effect of diabetes on sexual behavior and reproductive tract function in male rats. J Urol. 1993; 149:148–154.
Article31. Irshaid F, Mansi K. Effects of leaf extract of Urtica pilulifera L. on male reproductive system of streptozotocin-diabetic rats. Am J Pharmacol Toxicol. 2009; 4:22–28.
Article32. Mallick C, Mandal S, Barik B, Bhattacharya A, Ghosh D. Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol Pharm Bull. 2007; 30:84–90.
Article33. Tahtamouni LH, Alqurna NM, Al-Hudhud MY, Al-Hajj HA. Dandelion (Taraxacum officinale) decreases male rat fertility in vivo. J Ethnopharmacol. 2011; 135:102–109.
Article34. Mallidis C, Agbaje I, O'Neill J, McClure N. The influence of type 1 diabetes mellitus on spermatogenic gene expression. Fertil Steril. 2009; 92:2085–2087.
Article35. Hao J, Shen W, Sun L, Long J, Sharman E, Shi X, et al. Mitochondrial dysfunction in the liver of type 2 diabetic Goto-Kakizaki rats: improvement by a combination of nutrients. Br J Nutr. 2011; 106:648–655.
Article36. Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrinemale reproductive tract axis of the adult rat. J Urol. 1987; 138:190–194.
Article37. Padrón RS, Dambay A, Suárez R, Más J. Semen analyses in adolescent diabetic patients. Acta Diabetol Lat. 1984; 21:115–121.
Article38. O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996; 55:895–901.39. Shupe J, Cheng J, Puri P, Kostereva N, Walker WH. Regulation of Sertoli-germ cell adhesion and sperm release by FSH and nonclassical testosterone signaling. Mol Endocrinol. 2011; 25:238–252.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of sesame oil and different doses of estradiol on testicular structure, sperm parameters, and chromatin integrity in old mice
- Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice
- Testicular morphology and cauda epididymal sperm reserves of male rats exposed to Nigerian Qua Iboe Brent crude oil
- Effect of Melatonin on the Diabetes Mellitus Induced by Streptozotocin in Rats
- Dietary supplementation with astaxanthin may ameliorate sperm parameters and DNA integrity in streptozotocin-induced diabetic rats