Tuberc Respir Dis.
2013 Sep;75(3):95-103.
Neuroendocrine Differentiation in Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ccm@amc.seoul.kr
- 2Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
Abstract
- BACKGROUND
Small cell lung cancer (SCLC) transformation during epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) treatment in lung cancer has been suggested as one of possible resistance mechanisms.
METHODS
We evaluated whether SCLC transformation or neuroendocrine (NE) differentiation can be found in the cell line model. In addition, we also investigated its effect on responses to conventional chemotherapeutic drugs of the SCLC treatment.
RESULTS
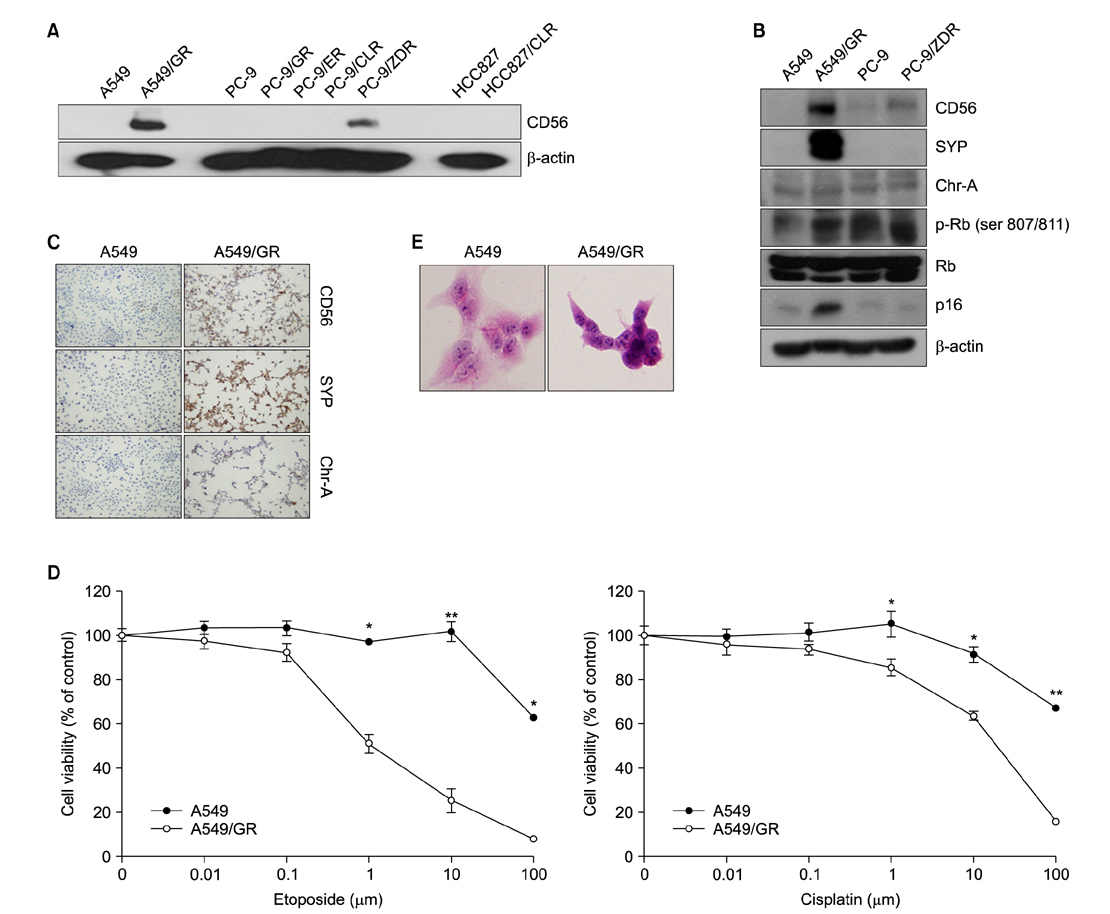
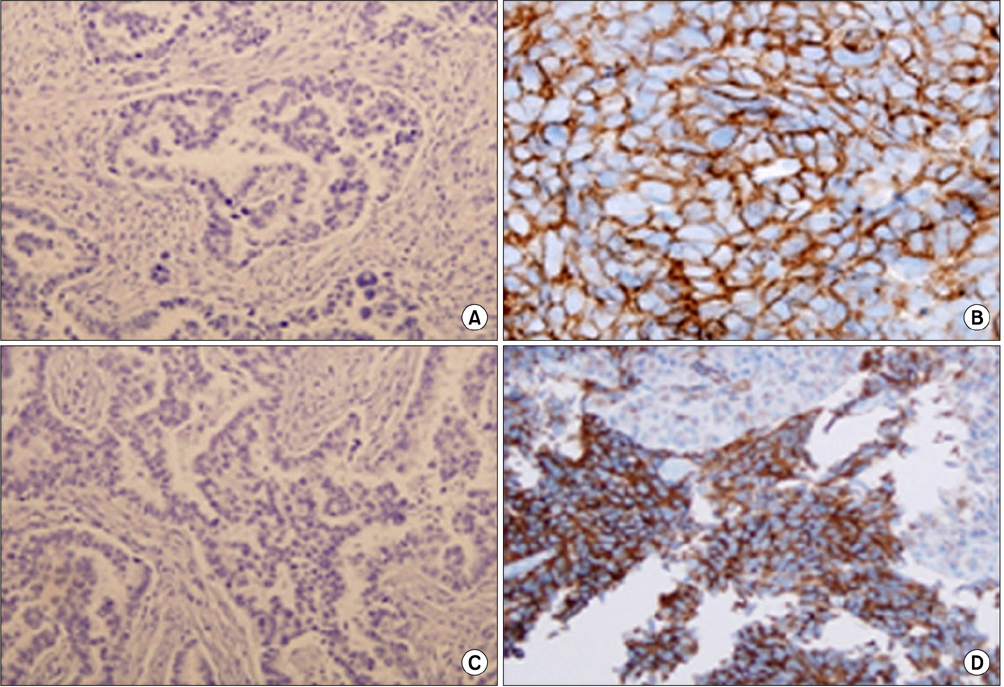
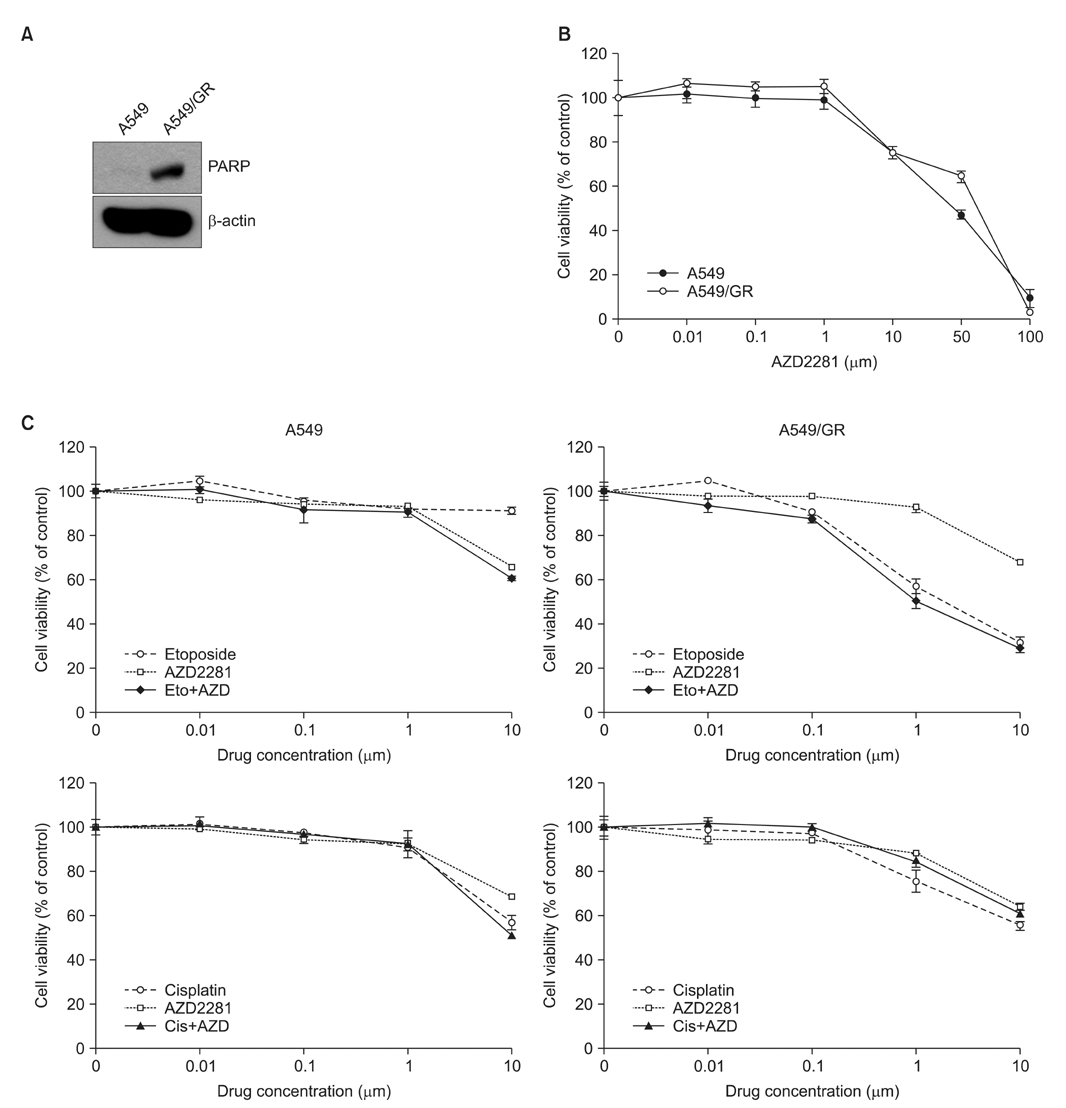
Resistant cell lines to various kinds of EGFR-TKIs such as gefitinib, erlotinib, CL-387,785 and ZD6474 with A549, PC-9 and HCC827 lung adenocarcinoma cell lines were established. Among them, two resistant cell lines, A549/GR (resistant to gefitinib) and PC-9/ZDR (resistant to ZD6474) showed increased expressions of CD56 while increased synaptophysin, Rb, p16 and poly(ADP-ribose) polymerase were found only in A549/GR in western blotting, suggesting that NE differentiation occurred in A549/GR. A549/GR cells were more sensitive to etoposide and cisplatin, chemotherapeutic drugs for SCLC, compared to parental cells. Treatment with cAMP and IBMX induced synaptophysin and chromogranin A expression in A549 cells, which also made them more sensitive to etoposide and cisplatin than parental cells. Furthermore, we found a tissue sample from a patient which showed increased expressions of CD56 and synaptophysin after development of resistance to erlotinib.
CONCLUSION
NE differentiation can occur during acquisition of resistance to EGFR-TKI, leading to increased chemosensitivity.
MeSH Terms
-
1-Methyl-3-isobutylxanthine
Adenocarcinoma
Blotting, Western
Cell Line
Cell Transformation, Neoplastic
Chromogranin A
Cisplatin
Drug Resistance, Neoplasm
Epidermal Growth Factor
Etoposide
Humans
Lung
Lung Neoplasms
Parents
Piperidines
Poly(ADP-ribose) Polymerases
Protein-Tyrosine Kinases
Quinazolines
Receptor, Epidermal Growth Factor
Small Cell Lung Carcinoma
Synaptophysin
Erlotinib Hydrochloride
1-Methyl-3-isobutylxanthine
Adenocarcinoma
Chromogranin A
Cisplatin
Epidermal Growth Factor
Etoposide
Lung Neoplasms
Piperidines
Poly(ADP-ribose) Polymerases
Protein-Tyrosine Kinases
Quinazolines
Receptor, Epidermal Growth Factor
Synaptophysin
Figure
Reference
-
1. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. Jama. 2003; 290:2149–2158.2. Perez-Soler R. Phase II clinical trial data with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (OSI-774) in non-small-cell lung cancer. Clin Lung Cancer. 2004; 6:Suppl 1. S20–S23.3. Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010; 28:357–360.4. Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011; 17:5530–5537.5. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005; 352:786–792.6. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005; 2:e73.7. Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005; 102:7665–7670.8. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012; 13:528–538.9. Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009; 462:1070–1074.10. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007; 316:1039–1043.11. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012; 44:852–860.12. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.13. Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007; 104:20932–20937.14. Morinaga R, Okamoto I, Furuta K, Kawano Y, Sekijima M, Dote K, et al. Sequential occurrence of non-small cell and small cell lung cancer with the same EGFR mutation. Lung Cancer. 2007; 58:411–413.15. Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, et al. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer. 2009; 63:219–226.16. Chung JH, Rho JK, Xu X, Lee JS, Yoon HI, Lee CT, et al. Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer. 2011; 73:176–182.17. Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, et al. The role of MET activation in determining the sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors. Mol Cancer Res. 2009; 7:1736–1743.18. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987; 47:936–942.19. Rho JK, Choi YJ, Ryoo BY, Na II, Yang SH, Kim CH, et al. p53 enhances gefitinib-induced growth inhibition and apoptosis by regulation of Fas in non-small cell lung cancer. Cancer Res. 2007; 67:1163–1169.20. Walker GE, Antoniono RJ, Ross HJ, Paisley TE, Oh Y. Neuroendocrine-like differentiation of non-small cell lung carcinoma cells: regulation by cAMP and the interaction of mac25/IGFBP-rP1 and 25.1. Oncogene. 2006; 25:1943–1954.21. Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012; 2:798–811.22. Tanno S, Ohsaki Y, Nakanishi K, Toyoshima E, Kikuchi K. Small cell lung cancer cells express EGFR and tyrosine phosphorylation of EGFR is inhibited by gefitinib ("Iressa", ZD1839). Oncol Rep. 2004; 12:1053–1057.23. Moore AM, Einhorn LH, Estes D, Govindan R, Axelson J, Vinson J, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: a Hoosier Oncology Group phase II trial. Lung Cancer. 2006; 52:93–97.24. Okamoto I, Araki J, Suto R, Shimada M, Nakagawa K, Fukuoka M. EGFR mutation in gefitinib-responsive small-cell lung cancer. Ann Oncol. 2006; 17:1028–1029.25. Zakowski MF, Ladanyi M, Kris MG. Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome Group. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006; 355:213–215.26. Sterlacci W, Fiegl M, Hilbe W, Auberger J, Mikuz G, Tzankov A. Clinical relevance of neuroendocrine differentiation in non-small cell lung cancer assessed by immunohistochemistry: a retrospective study on 405 surgically resected cases. Virchows Arch. 2009; 455:125–132.27. Carles J, Rosell R, Ariza A, Pellicer I, Sanchez JJ, Fernandez-Vasalo G, et al. Neuroendocrine differentiation as a prognostic factor in non-small cell lung cancer. Lung Cancer. 1993; 10:209–219.28. Graziano SL, Mazid R, Newman N, Tatum A, Oler A, Mortimer JA, et al. The use of neuroendocrine immunoperoxidase markers to predict chemotherapy response in patients with non-small-cell lung cancer. J Clin Oncol. 1989; 7:1398–1406.29. Gajra A, Tatum AH, Newman N, Gamble GP, Lichtenstein S, Rooney MT, et al. The predictive value of neuroendocrine markers and p53 for response to chemotherapy and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 2002; 36:159–165.30. Graziano SL, Kern JA, Herndon JE, Tatum A, Brisson ML, Memoli V, et al. Analysis of neuroendocrine markers, HER2 and CEA before and after chemotherapy in patients with stage IIIA non-small cell lung cancer: a Cancer and Leukemia Group B study. Lung Cancer. 1998; 21:203–211.31. Schleusener JT, Tazelaar HD, Jung SH, Cha SS, Cera PJ, Myers JL, et al. Neuroendocrine differentiation is an independent prognostic factor in chemotherapy-treated nonsmall cell lung carcinoma. Cancer. 1996; 77:1284–1291.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Mechanisms of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Resistance and Strategies to Overcome Resistance in Lung Adenocarcinoma
- Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor
- Mechanisms of Acquired Resistance to Epidermal Growth Factor Receptor Inhibitors and Overcoming Strategies in Lung Cancer
- Anaplastic lymphoma kinase (ALK)-expressing Lung Adenocarcinoma with Combined Neuroendocrine Component or Neuroendocrine Transformation: Implications for Neuroendocrine Transformation and Response to ALK-tyrosine Kinase Inhibitors