Korean J Urol.
2013 Dec;54(12):824-829.
Preoperative Factors Predictive of Posterolateral Extracapsular Extension After Radical Prostatectomy
- Affiliations
-
- 1Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hjahn@amc.seoul.kr
- 2Department of Urology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 3Department of Urology, Dankook University College of Medicine, Cheonan, Korea.
Abstract
- PURPOSE
In radical prostatectomy (RP) procedures, sparing the neurovascular bundles adjacent to the posterolateral aspect of the prostatic fascia has often been suggested as a possible risk factor for positive surgical margins. Here we aimed to quantify the probability of extracapsular extension (ECE) at the posterolateral side of the prostate to aid in nerve-sparing decision making.
MATERIALS AND METHODS
We evaluated 472 patients who underwent RP between July 2007 and January 2012. All patients underwent preoperative magnetic resonance imaging (MRI) with diffusion-weighted imaging and apparent diffusion coefficient mapping. We analyzed 944 side-specific prostate lobes with preoperative variables. To quantify the risk of side-specific posterolateral ECE after RP, we developed a risk-stratification scoring system through logistic regression analysis.
RESULTS
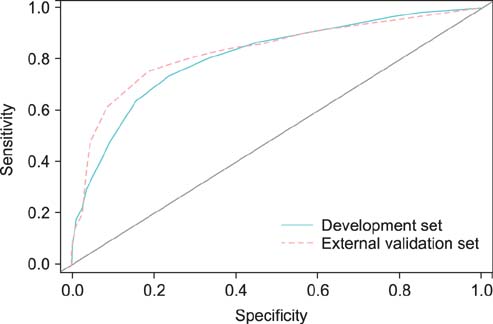
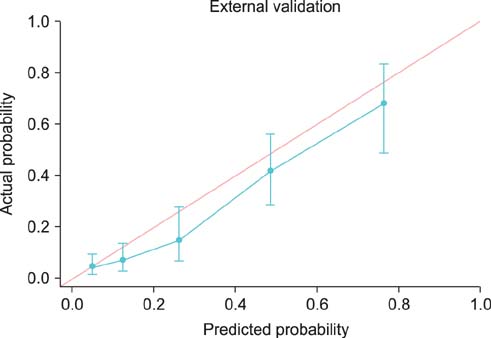
Overall, 20.6% of 944 prostate lobes had ECE. In the multivariate analysis, prostate-specific antigen (PSA), biopsy Gleason score > or =7, percentage of side-specific cores with tumor, and posterolateral ECE on MRI were independent predictive factors of posterolateral ECE. On internal and external validation to calculate the predicted risk, the Hosmer-Lemeshow goodness-of-fit test showed good calibration (p=0.396).
CONCLUSIONS
PSA, biopsy Gleason score, percentage of side-specific cores with tumor, and posterolateral ECE on MRI are independent predictors of posterolateral ECE. The scoring system derived from this study will provide objective parameters for use when deciding if the neurovascular bundle can be safely spared.
MeSH Terms
Figure
Reference
-
1. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012; 44:25–31.2. Chi BH, Chang IH. Prostate cancer: recent trends in Korea. Urol Int. 2010; 85:88–93.3. Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991; 145:998–1002.4. Berryhill R Jr, Jhaveri J, Yadav R, Leung R, Rao S, El-Hakim A, et al. Robotic prostatectomy: a review of outcomes compared with laparoscopic and open approaches. Urology. 2008; 72:15–23.5. Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009; 55:1037–1063.6. Raina R, Agarwal A, Zippe CD. Management of erectile dysfunction after radical prostatectomy. Urology. 2005; 66:923–929.7. Catalona WJ, Bigg SW. Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol. 1990; 143:538–543.8. Blute ML, Bostwick DG, Bergstralh EJ, Slezak JM, Martin SK, Amling CL, et al. Anatomic site-specific positive margins in organ-confined prostate cancer and its impact on outcome after radical prostatectomy. Urology. 1997; 50:733–739.9. Eastham JA, Kuroiwa K, Ohori M, Serio AM, Gorbonos A, Maru N, et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology. 2007; 70:965–969.10. Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005; 66:1245–1250.11. Katz R, Salomon L, Hoznek A, de la Taille A, Antiphon P, Abbou CC. Positive surgical margins in laparoscopic radical prostatectomy: the impact of apical dissection, bladder neck remodeling and nerve preservation. J Urol. 2003; 169:2049–2052.12. Nelles JL, Freedland SJ, Presti JC Jr, Terris MK, Aronson WJ, Amling CL, et al. Impact of nerve sparing on surgical margins and biochemical recurrence: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2009; 12:172–176.13. Palisaar RJ, Noldus J, Graefen M, Erbersdobler A, Haese A, Huland H. Influence of nerve-sparing (NS) procedure during radical prostatectomy (RP) on margin status and biochemical failure. Eur Urol. 2005; 47:176–184.14. Sofer M, Hamilton-Nelson KL, Civantos F, Soloway MS. Positive surgical margins after radical retropubic prostatectomy: the influence of site and number on progression. J Urol. 2002; 167:2453–2456.15. Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, Blute ML. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004; 172(4 Pt 1):1328–1332.16. Liss M, Osann K, Ornstein D. Positive surgical margins during robotic radical prostatectomy: a contemporary analysis of risk factors. BJU Int. 2008; 102:603–608.17. Potdevin L, Ercolani M, Jeong J, Kim IY. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol. 2009; 23:1479–1484.18. Shikanov S, Woo J, Al-Ahmadie H, Katz MH, Zagaja GP, Shalhav AL, et al. Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: comparison of functional outcomes and positive surgical margins characteristics. Urology. 2009; 74:611–616.19. Smith JA Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007; 178:2385–2389.20. Graefen M, Haese A, Pichlmeier U, Hammerer PG, Noldus J, Butz K, et al. A validated strategy for side specific prediction of organ confined prostate cancer: a tool to select for nerve sparing radical prostatectomy. J Urol. 2001; 165:857–863.21. Shah O, Robbins DA, Melamed J, Lepor H. The New York University nerve sparing algorithm decreases the rate of positive surgical margins following radical retropubic prostatectomy. J Urol. 2003; 169:2147–2152.22. Tewari AK, Srivastava A, Huang MW, Robinson BD, Shevchuk MM, Durand M, et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 2011; 108(6 Pt 2):984–992.23. Tsuzuki T, Hernandez DJ, Aydin H, Trock B, Walsh PC, Epstein JI. Prediction of extraprostatic extension in the neurovascular bundle based on prostate needle biopsy pathology, serum prostate specific antigen and digital rectal examination. J Urol. 2005; 173:450–453.24. Stephenson AJ, Wood DP, Kattan MW, Klein EA, Scardino PT, Eastham JA, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009; 182:1357–1363.25. Lepor H, Tareen B. Neurovascular bundle resection: does it improve the margins? Urol Oncol. 2010; 28:215–218.26. McClure TD, Margolis DJ, Reiter RE, Sayre JW, Thomas MA, Nagarajan R, et al. Use of MR imaging to determine preservation of the neurovascular bundles at robotic-assisted laparoscopic prostatectomy. Radiology. 2012; 262:874–883.27. Bloch BN, Furman-Haran E, Helbich TH, Lenkinski RE, Degani H, Kratzik C, et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging: initial results. Radiology. 2007; 245:176–185.28. Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006; 238:597–603.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ability of Core Biopsies to Predict Extracapsular Extension of Prostate Cancer
- Prostate-Specific Antigen Density as a Powerful Predictor of Extracapsular Extension and Positive Surgical Margin in Radical Prostatectomy Patients with Prostate-Specific Antigen Levels of Less than 10 ng/ml
- The Pathological Characteristics of Localized Prostate Cancer, Managed with Radical Prostatectomy
- pT3 Predictive Factors in Patients with a Gleason Score of 6 in Prostate Biopsies
- Preoperative Nomograms for Predicting Extracapsular Extension in Korean Men with Localized Prostate Cancer: A Multi-institutional Clinicopathologic Study