Korean J Urol.
2011 Apr;52(4):253-259.
Cyclooxygenase-2 Overexpression in Chronic Inflammation Associated with Benign Prostatic Hyperplasia: Is It Related to Apoptosis and Angiogenesis of Prostate Cancer?
- Affiliations
-
- 1Department of Urology, Keimyung University School of Medicine, Daegu, Korea. chp@dsmc.or.kr
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Urology, College of Medicine, Daegu Catholic University, Daegu, Korea.
Abstract
- PURPOSE
This study was performed to investigate the relationship between cyclooxygenase-2 (COX-2) expression and apoptosis/angiogenesis in inflammatory and noninflammatory benign prostatic hyperplasia (BPH) and prostate cancer (PC).
MATERIALS AND METHODS
This study involved 64 BPH and 57 PC patients. The BPH histopathologies were classified by the presence of chronic inflammation as follows: noninflammatory BPH (NI-BPH; n=23) and inflammatory BPH (I-BPH; n=41). The association between the expression of COX-2, expression of Bcl-2, the apoptotic index (AI), expression of vascular endothelial growth factor (VEGF), and microvascular density (MVD) in the prostate was investigated.
RESULTS
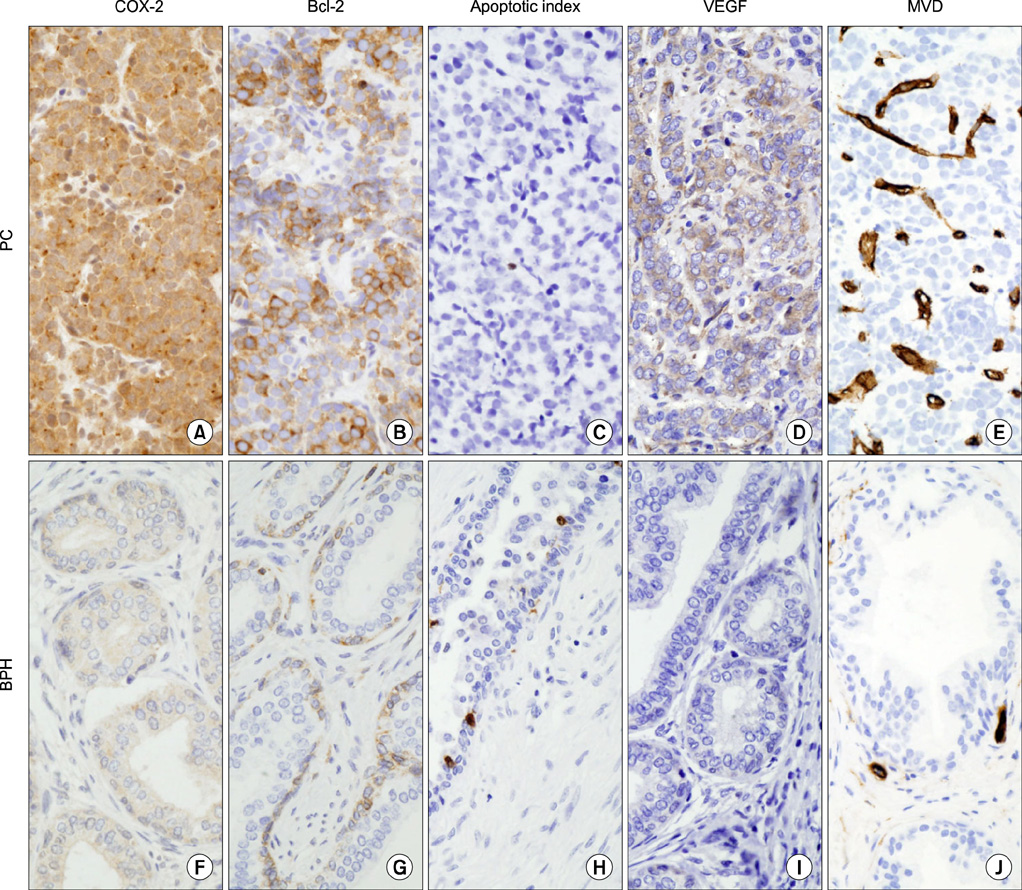
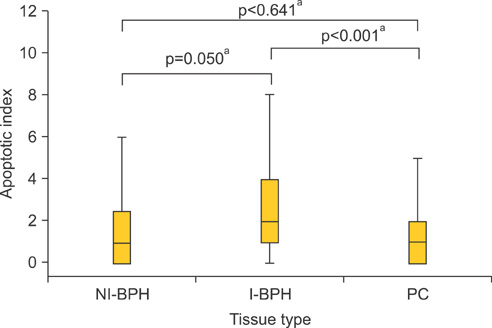
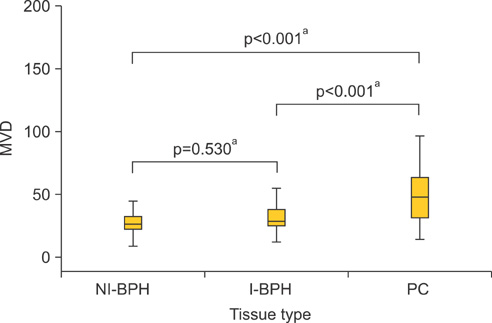
An overexpression of COX-2, Bcl-2, and VEGF was observed in cases of PC compared with cases of BPH. In PC, the AI was lower and MVD was higher than in BPH. In NI-BPH, I-BPH, and PC, the overexpression of COX-2, Bcl-2, and VEGF gradually increased. The AI was high in I-BPH, but did not differ significantly between the NI-BPH and I-BPH groups or between the NI-BPH and PC groups. MVD was significantly high in PC, but no significant difference was found between NI-BPH and I-BPH. A significant correlation was shown between the overexpression of COX-2 and Bcl-2, and COX-2 and VEGF. However, the AI was not correlated with the overexpression of COX-2 or Bcl-2. MVD was correlated with the overexpression of COX-2 and VEGF.
CONCLUSIONS
COX-2 overexpression in PC is correlated with a decrease in apoptosis and an increase in angiogenesis. Chronic inflammation in BPH causes an overexpression of COX-2, which induces the increased expression of Bcl-2 and VEGF. It is likely that chronic inflammation plays a role in the intermediate step of carcinogenesis in the prostate.
Keyword
MeSH Terms
Figure
Reference
-
1. Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000. 85:60–67.2. Roberts MJ, Schirra HJ, Lavin MF, Gardiner RA. Metabolomics: a novel approach to early and noninvasive prostate cancer detection. Korean J Urol. 2011. 52:79–89.3. De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007. 7:256–269.4. Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000. 56:671–676.5. Madaan S, Abel PD, Chaudhary KS, Hewitt R, Stott MA, Stamp GW, et al. Cytoplasmic induction and over-expression of cyclooxygenase-2 in human prostate cancer: implications for prevention and treatment. BJU Int. 2000. 86:736–741.6. Chu J, Lloyd FL, Trifan OC, Knapp B, Rizzo MT. Potential involvement of the cyclooxygenase-2 pathway in the regulation of tumor-associated angiogenesis and growth in pancreatic cancer. Mol Cancer Ther. 2003. 2:1–7.7. Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, et al. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003. 23:1317–1322.8. Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998. 58:4245–4249.9. Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000. 89:589–596.10. Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, et al. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001. 61:8617–8623.11. Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004. 61:60–72.12. Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005. 11:3250–3256.13. Denkert C, Thoma A, Niesporek S, Weichert W, Koch I, Noske A, et al. Overexpression of cyclooxygenase-2 in human prostate carcinoma and prostatic intraepithelial neoplasia-association with increased expression of Polo-like kinase-1. Prostate. 2007. 67:361–369.14. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992. 119:493–501.15. Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biol. 2004. 5:241.16. Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002. 3:166–174.17. Dai Y, Wang WH. Non-steroidal anti-inflammatory drugs in prevention of gastric cancer. World J Gastroenterol. 2006. 12:2884–2889.18. Scartozzi M, Galizia E, Freddari F, Berardi R, Cellerino R, Cascinu S. Molecular biology of sporadic gastric cancer: prognostic indicators and novel therapeutic approaches. Cancer Treat Rev. 2004. 30:451–459.19. Dimmeler S, Breitschopf K, Haendeler J, Zeiher AM. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J Exp Med. 1999. 189:1815–1822.20. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995. 146:3–15.21. Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010. 2010:215158.22. Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998. 95:681–686.23. Chávez-Galán L, Arenas-Del Angel MC, Zenteno E, Chávez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009. 6:15–25.24. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987. 235:442–447.25. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998. 93:705–716.26. Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000. 60:1306–1311.27. Fujiwaki R, Iida K, Kanasaki H, Ozaki T, Hata K, Miyazaki K. Cyclooxygenase-2 expression in endometrial cancer: correlation with microvessel count and expression of vascular endothelial growth factor and thymidine phosphorylase. Hum Pathol. 2002. 33:213–219.28. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003. 9:669–676.29. Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001. 3:53–61.30. Zhi YH, Liu RS, Song MM, Tian Y, Long J, Tu W, et al. Cyclooxygenase-2 promotes angiogenesis by increasing vascular endothelial growth factor and predicts prognosis in gallbladder carcinoma. World J Gastroenterol. 2005. 11:3724–3728.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis and Expression of p53, bcl-2 and c-myc Proteins in Benign Prostatic Hyperplasia and Prostate Cancer
- Expression of Survivin Correlated with Antiapoptosis in Benign Prostate Hyperplasia and Prostate Cancer
- Histopathologic Study of non-Cancerous Lesion of the Prostate
- Induction of Prostate Apoptosis by Low Dose Terazosin in Benign Prostatic Hyperplasia
- The role of serum prostate specific antigen in prostatic cancer and benign prostatic hyperplasia