Korean J Urol.
2009 Jul;50(7):704-710.
The Different Effects of Testicular Torsion on the Contralateral Testis between Pubertal and Adult Rats
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Urology, Seoul National University College of Medicine, Seoul, Korea. hchoi@snu.ac.kr
- 3Department of Urology, Pochon CHA University Bundang CHA Hospital, Seongnam, Korea.
- 4Department of Urology, Konkuk University Medical Center, Seoul, Korea.
Abstract
- PURPOSE
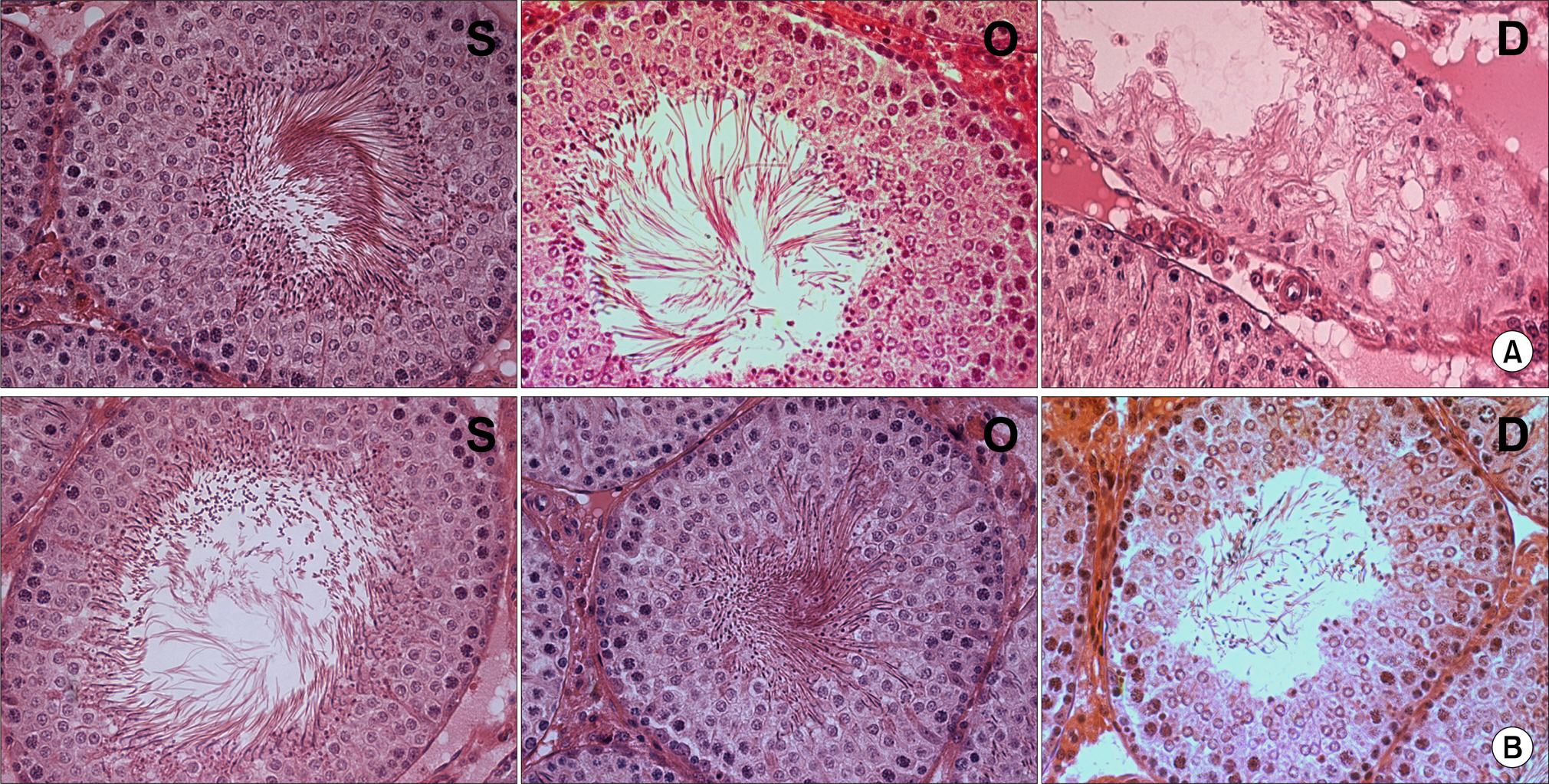
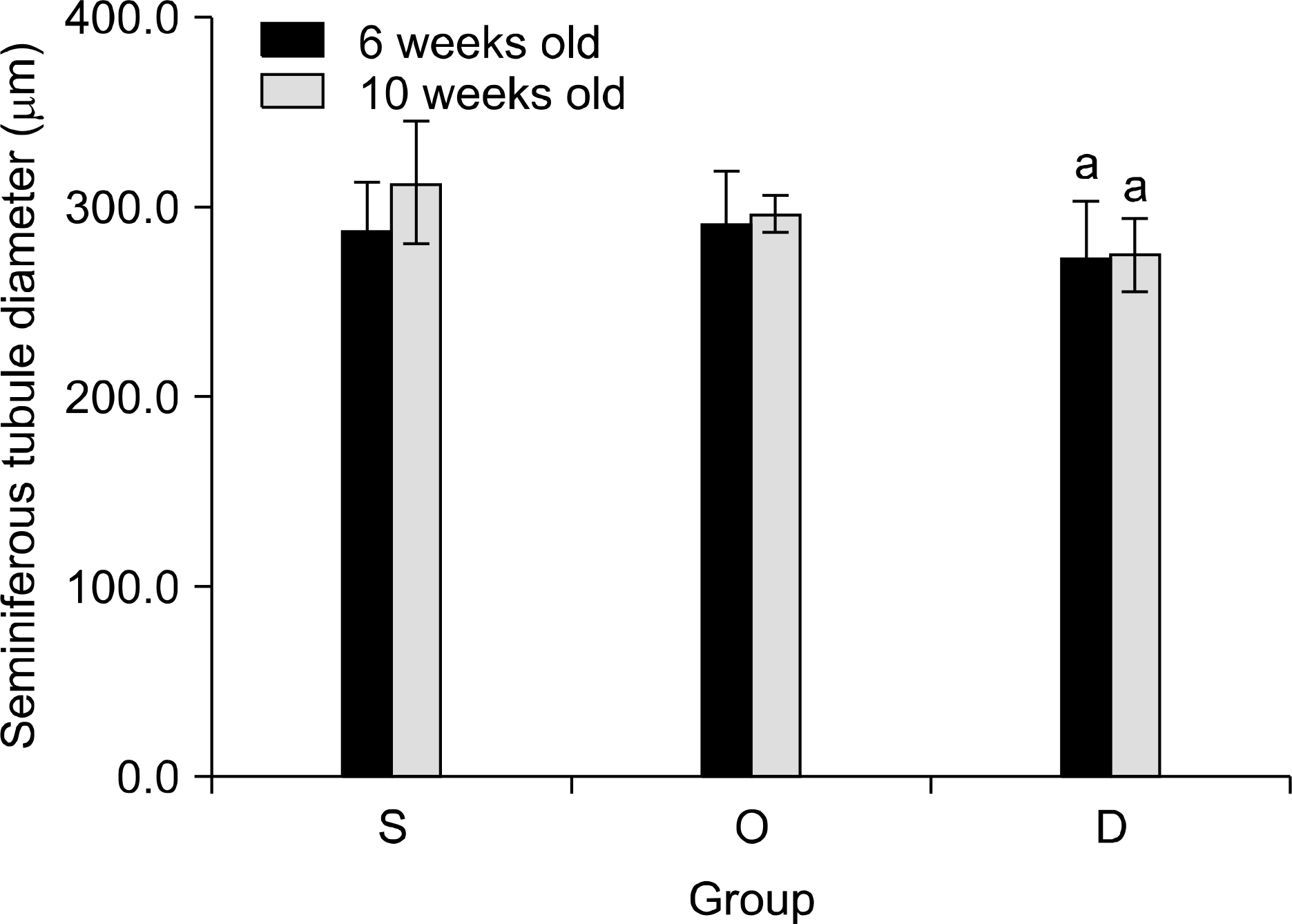
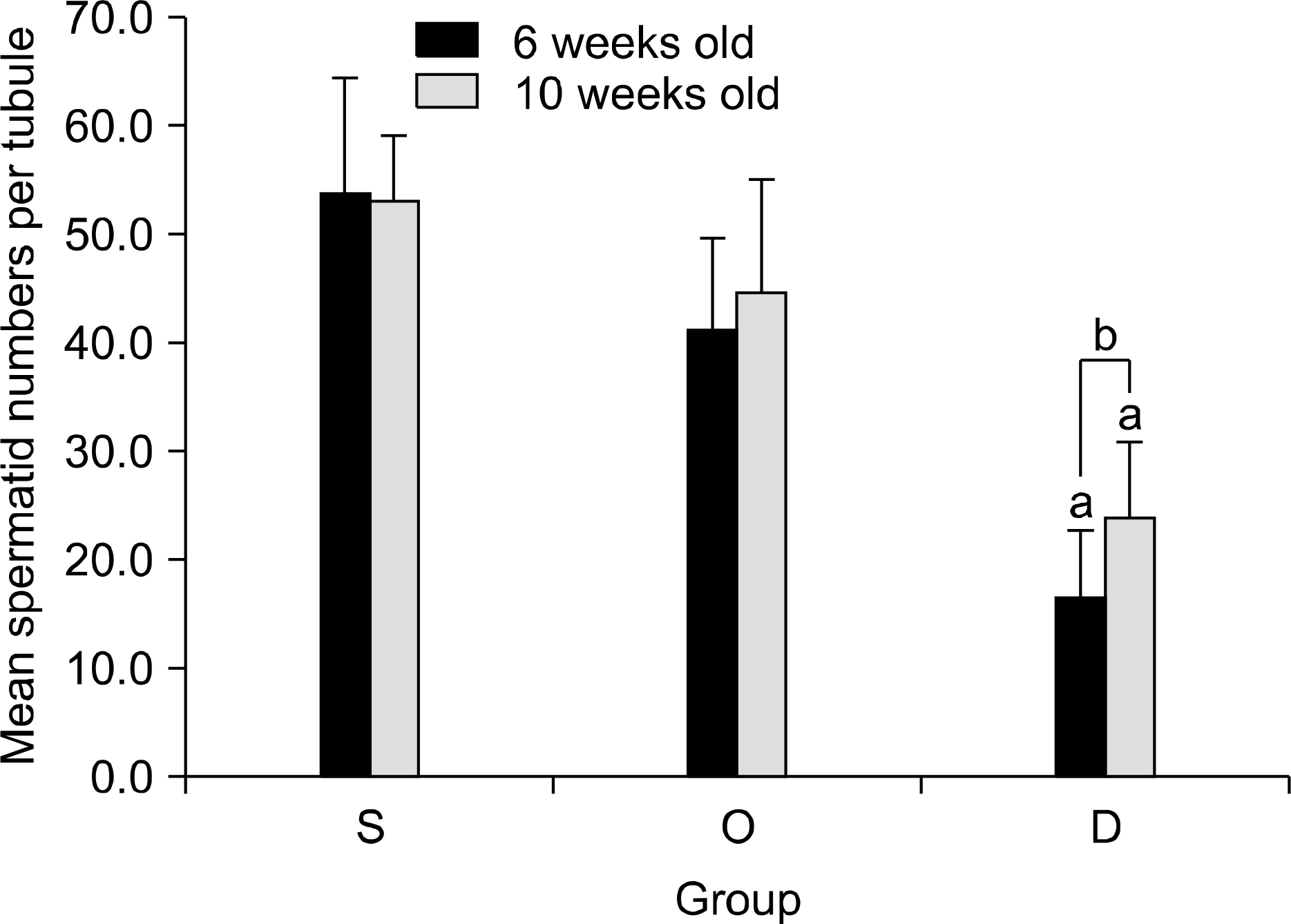
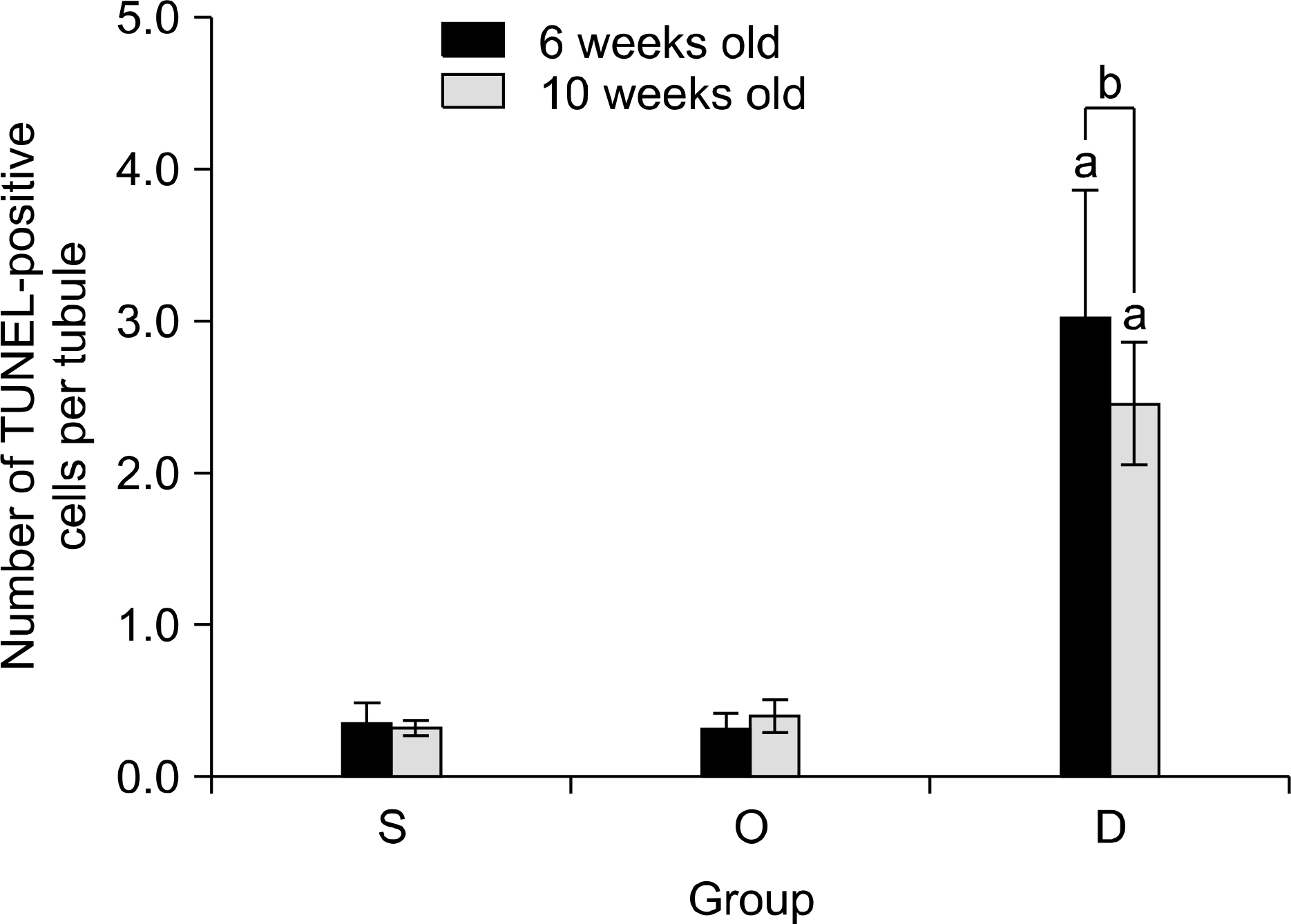
Our study aimed to determine whether the severity of damage to the contralateral testis by ipsilateral testicular torsion/detorsion in pubertal rats, which have an incomplete blood-testis barrier, is different from that in adult rats. MATERIALS AND METHODS: We divided pubertal (6 weeks, n=17) and adult (10 weeks, n=17) Sprague-Dawley (SD) rats into group S (sham; n=5), group O (orchiectomy; n=6), and group D (detorsion; n=6). After 4 hours' torsion of the ipsilateral testis, we applied orchiectomy (group O) and detorsion (group D) depending on the group and compared the histopathologic changes and germ cell apoptosis of the contralateral testis at the age of 13 weeks. RESULTS: In each age group, increased interstitial area, edema, and germ cell sloughing were observed in group D. The mean seminiferous tubule diameter decreased more in group D than in group S or O in each age group (p<0.05). The mean germ cell layer thickness and number of spermatids per tubule decreased more in group D than in group S or O in each age group; additionally, in group D, values decreased more in pubertal rats than in adult ones (p<0.05, respectively). The mean numbers of terminal deoxyuridine nick-end labeling (TUNEL)-positive cells were less than 1.0 in groups S and O, which was smaller than in group D (p<0.05); additionally, in group D, this value tended to be higher in pubertal rats than in adult ones (p=0.057). CONCLUSIONS: SD rats with a detorsioned testis had more severe damage to the contralateral testis than did those undergoing orchiectomy of the torsioned testis. Also, when comparing the severity of damage to the contralateral testis after ipsilateral torsion/detorsion between pubertal and adult rats, rats at a pubertal age, when most testicular torsions occur in clinical situations, had more severe damage than did those at an adult age.
Keyword
MeSH Terms
Figure
Reference
-
1.Melekos MD., Asbach HW., Markou SA. Etiology of acute scrotum in 100 boys with regard to age distribution. J Urol. 1988. 139:1023–5.
Article2.Krarup T. The testes after torsion. Br J Urol. 1978. 50:43–6.
Article3.Williamson RC. Torsion of the testis and allied conditions. Br J Surg. 1976. 63:465–76.
Article4.Anderson JB., Williamson RC. The fate of the human testes following unilateral torsion of the spermatic cord. Br J Urol. 1986. 58:698–704.
Article5.Akgür FM., Kilinc K., Aktug T. Reperfusion injury after detorsion of unilateral testicular torsion. Urol Res. 1993. 21:395–9.
Article6.Cerasaro TS., Nachtsheim DA., Otero F., Parsons CL. The effect of testicular torsion on the contralateral testis and the production of antisperm antibodies in rabbits. J Urol. 1984. 132:577–9.7.Kosar A., Sarica K., Kupeli B., Alçiǧir G., Süzer O., Küpeli S. Testicular torsion: evaluation of contralateral testicular histology. Int Urol Nephrol. 1997. 29:351–6.8.Hadziselimovic F., Geneto R., Emmons LR. Increased apoptosis in the contralateral testis in patients with testicular torsion. Lancet. 1997. 350:118.
Article9.Turner TT. Acute experimental testicular torsion. No effect on the contralateral testis. J Androl. 1985. 6:65–72.
Article10.Becker EJ Jr., Turner TT. Endocrine and exocrine effects of testicular torsion in the prepubertal and adult rat. J Androl. 1995. 16:342–51.11.Gang JW., Yang KY. Comparisons of the effects of testicular torsion through a scrotal wall incision and an abdominal wall incision on the contralateral testis in prepubertal rats. Korean J Urol. 1992. 33:816–21.12.Clermont Y., Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat. 1957. 100:241–67.
Article13.Steinberger A., Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod. 1971. 4:84–7.14.Heindel RM., Pakyz RE., Consentino MJ. Spermatic cord torsion. Contralateral testicular degeneration at various ages in the rat. J Androl. 1990. 11:506–13.15.Shin HS., Cho CK., Moon KH. The effect of testicular torsion on the histologic findings and apoptosis of the contralateral testis at various ages in rats. Korean J Urol. 2002. 43:693–8.16.Nagler HM., White RD. The effect of testicular torsion on the contralateral testis. J Urol. 1982. 128:1343–8.
Article17.Sarica K., Kupeli B., Budak M., Koşar A., Kavukçu M., Durak I, et al. Influence of experimental spermatic cord torsion on the contralateral testis in rats. Evaluation of tissue free oxygen radical scavenger enzyme levels. Urol Int. 1997. 58:208–12.18.Park EC., Kwak C., Son H., Oh SJ., Lee SE., Choi H. The role of clusterin in apoptosis induced by testicular torsion. Korean J Urol. 2004. 45:380–9.19.Turner TT., Tung KS., Tomomasa H., Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997. 57:1267–74.
Article20.Werns SW., Lucchesi BR. Free radicals and ischemic tissue injury. Trends Pharmacol Sci. 1990. 11:161–6.
Article21.Chemes HE., Dym M., Fawcett DW., Javadpour N., Sherins RJ. Patho-physiological observations of Sertoli cells in patients with germinal aplasia or severe germ cell depletion. Ultrastructural findings and hormone levels. Biol Reprod. 1977. 17:108–23.22.Russell LD., Malone JP. A study of Sertoli-spermatid tubulo-bulbar complexes in selected mammals. Tissue Cell. 1980. 12:263–85.
Article23.Hadziselimovic F., Geneto R., Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998. 160:1158–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of testicular torsion on the contralateral testis in rats
- The Effect of Testicular Torsion on the Contralateral Testis in Rats; Histologic Changes and its Reversibility
- The Effect of Testicular Torsion on the Histologic Findings and Apoptosis of the Contralateral Testis at Various Ages in Rats
- Protective Effect of Capsaicin on Contralateral Testis of Rats during Unilateral Testicular Torsion
- The Role of Clusterin in Apoptosis Induced by Testicular Torsion