Nutr Res Pract.
2015 Jun;9(3):235-241. 10.4162/nrp.2015.9.3.235.
Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet
- Affiliations
-
- 1Department of Food and Nutrition, Seoul National University, Seoul 151-742, Korea. hye0414@snu.ac.kr
- 2Research Institute of Human Ecology, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea.
- 3Department of Biotechnology, Hoseo University, Asan 336-795, Korea.
- 4Institute on Aging, Seoul National University, Seoul 110-799, Korea.
- KMID: 2313835
- DOI: http://doi.org/10.4162/nrp.2015.9.3.235
Abstract
- BACKGROUND/OBJECTIVES
Doenjang, Korean traditional fermented soybean paste has been reported to have an anti-obesity effect. Because adipose tissue is considered a major source of inflammatory signals, we investigated the protective effects of Doenjang and steamed soybean on oxidative stress and inflammation in adipose tissue of diet-induced obese mice.
MATERIALS/METHODS
Male C57BL/6J mice were fed a low fat diet (LF), a high-fat diet (HF), or a high-fat containing Doenjang diet (DJ) or a high-fat containing steamed soybean diet (SS) for 11 weeks.
RESULTS
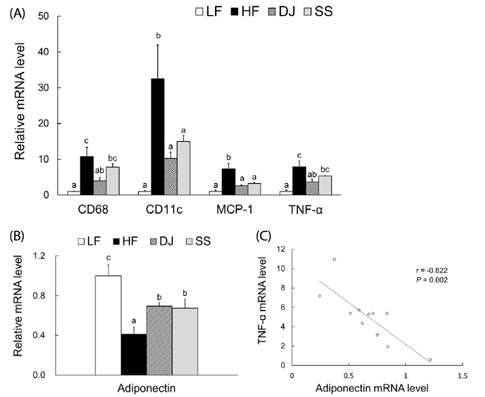
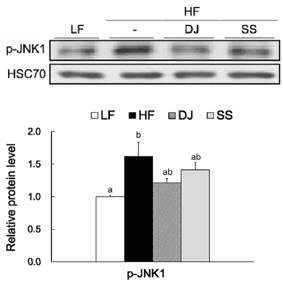
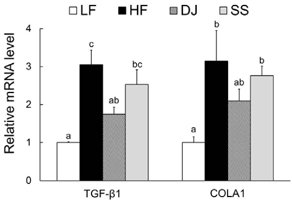
Mice fed a DJ diet showed significantly lower body and adipose tissue weights than those in the HF group. Although no significant differences in adipocyte size and number were observed among the HF diet-fed groups, consumption of Doenjang alleviated the incidence of crown-like structures in adipose tissue. Consistently, we observed significantly reduced mRNA levels of oxidative stress markers (heme oxygenase-1 and p40phox), pro-inflammatory adipokines (tumor necrosis factor alpha and macrophage chemoattractant protein-1), macrophage markers (CD68 and CD11c), and a fibrosis marker (transforming growth factor beta 1) by Doenjang consumption. Gene expression of anti-inflammatory adipokine, adiponectin was significantly induced in the DJ group and the SS group compared to the HF group. The anti-oxidative stress and anti-inflammatory effects observed in mice fed an SS diet were not as effective as those in mice fed a DJ diet, suggesting that the bioactive compounds produced during fermentation and aging may be involved in the observed health-beneficial effects of Doenjang.
CONCLUSIONS
Doenjang alleviated oxidative stress and restored the dysregulated expression of adipokine genes caused by excess adiposity. Therefore, Doenjang may ameliorate systemic inflammation and oxidative stress in obesity via inhibition of inflammatory signals of adipose tissue.
Keyword
MeSH Terms
-
Adipocytes
Adipokines
Adiponectin
Adipose Tissue*
Adiposity
Aging
Animals
Diet
Diet, High-Fat*
Fermentation
Fibrosis
Gene Expression
Humans
Incidence
Inflammation*
Macrophages
Male
Mice*
Mice, Obese
Necrosis
Obesity
Oxidative Stress*
RNA, Messenger
Soybeans*
Steam
Weights and Measures
Adipokines
Adiponectin
RNA, Messenger
Steam
Figure
Cited by 1 articles
-
Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice
Ji-Hyun Oh, Jaehoon Kim, Yunkyoung Lee
Nutr Res Pract. 2016;10(1):42-48. doi: 10.4162/nrp.2016.10.1.42.
Reference
-
1. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85–97.
Article2. Harwood HJ Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012; 63:57–75.
Article3. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010; 314:1–16.
Article4. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
Article5. Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005; 327:460–467.
Article6. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001; 50:2094–2099.
Article7. Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012; 32:261–286.
Article8. Kwon DY, Daily JW 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010; 30:1–13.
Article9. Chai C, Ju HK, Kim SC, Park JH, Lim J, Kwon SW, Lee J. Determination of bioactive compounds in fermented soybean products using GC/MS and further investigation of correlation of their bioactivities. J Chromatogr B Analyt Technol Biomed Life Sci. 2012; 880:42–49.
Article10. Chung SI, Rico CW, Kang MY. Comparative study on the hypoglycemic and antioxidative effects of fermented paste (doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients. 2014; 6:4610–4624.
Article11. Jung KO, Park SY, Park KY. Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition. 2006; 22:539–545.
Article12. Park KY, Jung KO, Rhee SH, Choi YH. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res. 2003; 523-524:43–53.
Article13. Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012; 16:1295–1322.
Article14. Kwak CS, Park SC, Song KY. Doenjang, a fermented soybean paste, decreased visceral fat accumulation and adipocyte size in rats fed with high fat diet more effectively than nonfermented soybeans. J Med Food. 2012; 15:1–9.
Article15. Cha YS, Yang JA, Back HI, Kim SR, Kim MG, Jung SJ, Song WO, Chae SW. Visceral fat and body weight are reduced in overweight adults by the supplementation of Doenjang, a fermented soybean paste. Nutr Res Pract. 2012; 6:520–526.
Article16. Fang N, Yu S, Badger TM. Comprehensive phytochemical profile of soy protein isolate. J Agric Food Chem. 2004; 52:4012–4020.
Article17. Jeon S, Park YJ, Kwon YH. Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(-/-) mice fed a high-fat diet. Mol Nutr Food Res. 2014; 58:830–841.
Article18. Ji G, Yang Q, Hao J, Guo L, Chen X, Hu J, Leng L, Jiang Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol. 2011; 11:762–768.
Article19. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007; 87:245–313.
Article20. Boschmann M, Engeli S, Adams F, Gorzelniak K, Franke G, Klaua S, Kreuzberg U, Luedtke S, Kettritz R, Sharma AM, Luft FC, Jordan J. Adipose tissue metabolism and CD11b expression on monocytes in obese hypertensives. Hypertension. 2005; 46:130–136.
Article21. Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010; 285:15333–15345.
Article22. Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008; 582:117–131.23. Bae CR, Kwon DY, Cha YS. Anti-obesity effects of traditional and standardized meju in high-fat diet-induced obese C57BL/6J mice. J Clin Biochem Nutr. 2014; 54:45–50.
Article24. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article25. Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, Dusting GJ. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. 2011; 16:223–229.
Article26. Nickelson KJ, Stromsdorfer KL, Pickering RT, Liu TW, Ortinau LC, Keating AF, Perfield JW 2nd. A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Exp Diabetes Res. 2012; 2012:859395.
Article27. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006; 86:583–650.
Article28. Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999; 13:1231–1238.
Article29. Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, Smirčić Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014; 20:18070–18091.
Article30. Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong ML, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol. 2012; 56:597–607.
Article31. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007; 380:24–30.
Article32. Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013; 18:470–477.
Article33. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–827.34. Liu XJ, Yang L, Mao YQ, Wang Q, Huang MH, Wang YP, Wu HB. Effects of the tyrosine protein kinase inhibitor genistein on the proliferation, activation of cultured rat hepatic stellate cells. World J Gastroenterol. 2002; 8:739–745.
Article35. Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011; 60:1246–1257.
Article36. Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010; 59:2817–2825.
Article37. Nagaraju GP, Zafar SF, El-Rayes BF. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr Rev. 2013; 71:562–572.
Article38. Omoni AO, Aluko RE. Soybean foods and their benefits: potential mechanisms of action. Nutr Rev. 2005; 63:272–283.
Article39. Korhonen H, Pihlanto A. Food-derived bioactive peptides--opportunities for designing future foods. Curr Pharm Des. 2003; 9:1297–1308.40. Kwon DY, Hong SM, Ahn IS, Kim MJ, Yang HJ, Park S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition. 2011; 27:244–252.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of caloric restriction on the expression of lipocalin-2 and its receptor in the brown adipose tissue of high-fat diet-fed mice
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet
- Anti-obesity Effect of Steamed Soybean and Fermented Steamed Soybean in High-fat Diet-induced Obese ICR Mice
- Effects of disturbed liver growth and oxidative stress of high-fat diet-fed dams on cholesterol metabolism in offspring mice
- Effects of Toll-like receptor antagonist 4,5-dihydro-3-phenyl-5-isoxasole acetic acid on the progression of kidney disease in mice on a high-fat diet