Nutr Res Pract.
2012 Apr;6(2):97-105.
Suppressive effects of Schizandra chinensis Baillon water extract on allergy-related cytokine generation and degranulation in IgE-antigen complex-stimulated RBL-2H3 cells
- Affiliations
-
- 1The Nutraceutical Bio Brain Korea 21 Project Group, Kangwon National University, Chuncheon 200-701, Korea.
- 2Divison of Bio-Health Technology, College of Biomedical Science, Kangwon National University, 1 Kangwondaehak-gil, Chuncheon, Gangwon 200-701, Korea. mchoe@kangwon.ac.kr
- 3Corporate-affiliated Research Institute of Academic-Industrial-Institutional Cooperation, Borinara Co. LTD., Chuncheon 200-870, Korea.
- 4Well-Being Bioproducts RIC Center, Kangwon National University, Gangwon 200-701, Korea.
- 5Department of Food and Nutrition, Baewha Women's University, Seoul 110-735, Korea.
Abstract
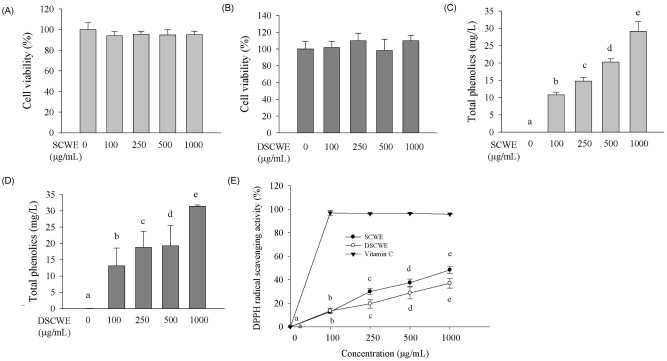
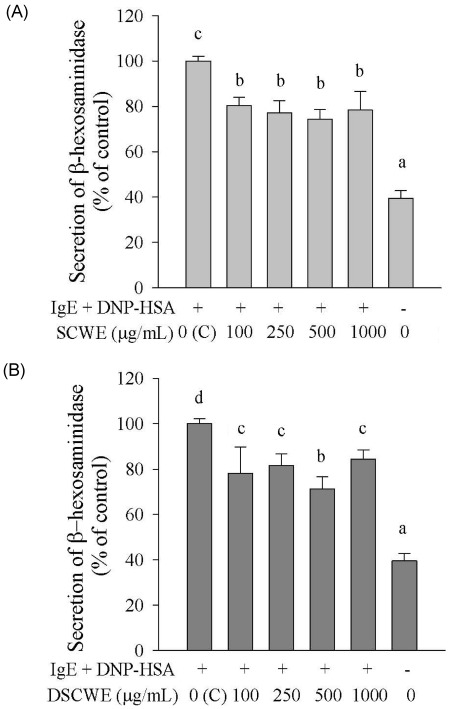
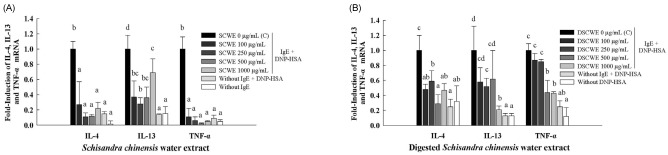
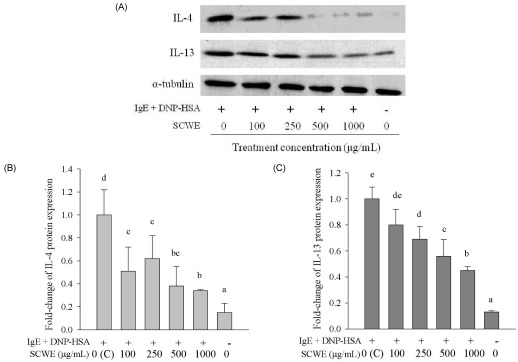
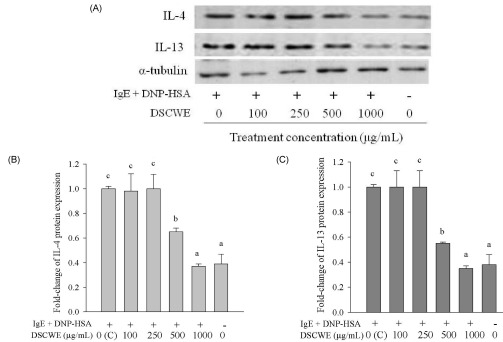
- Schizandra chinensis Baillon is a traditional folk medicine plant that is used to treat and prevent several inflammatory diseases and cancer in Korea, but the underlying mechanisms involved in its anti-allergic activity are not fully understood. This study was designed to investigate mechanisms of anti-allergic activity of a Schizandra chinensis Baillon water extract (SCWE) in immunoglobulin E (IgE)-antigen complex-stimulated RBL2H3 cells and to assess whether gastric and intestinal digestion affects the anti-allergic properties of SCWE. Oxidative stress is an important consequence of the allergic inflammatory response. The antioxidant activities of SCWE increased in a concentration-dependent manner. RBL-2H3 cells were sensitized with monoclonal anti-dinitrophenol (DNP) specific IgE, treated with SCWE, and challenged with the antigen DNP-human serum albumin. SCWE inhibited beta-hexosaminidase release and expression of interleukin (IL)-4, IL-13, and tumor necrosis factor-alpha (TNF-alpha) mRNA and protein in IgE-antigen complex-stimulated RBL2H3 cells. We found that digested SCWE fully maintained its antioxidant activity and anti-allergic activity against the IgE-antigen complex-induced activation of RBL-2H3 cells. SCWE may be useful for preventing allergic diseases, such as asthma. Thus, SCWE could be used as a natural functional ingredient for allergic diseases in the food and/or pharmaceutical industries.
MeSH Terms
-
Asthma
beta-N-Acetylhexosaminidases
Digestion
Drug Industry
Immunoglobulin E
Immunoglobulins
Interleukin-13
Interleukins
Korea
Medicine, Traditional
Oxidative Stress
Plants
RNA, Messenger
Schisandra
Serum Albumin
Tumor Necrosis Factor-alpha
Water
Immunoglobulin E
Immunoglobulins
Interleukin-13
Interleukins
RNA, Messenger
Serum Albumin
Tumor Necrosis Factor-alpha
Water
beta-N-Acetylhexosaminidases
Figure
Reference
-
1. Itoh T, Ohguchi K, Iinuma M, Nozawa Y, Akao Y. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg Med Chem. 2008; 16:4500–4508. PMID: 18328716.2. Matsuda H, Wang Q, Matsuhira K, Nakamura S, Yuan D, Yoshikawa M. Inhibitory effects of thunberginols A and B isolated from Hydrangeae Dulcis Folium on mRNA expression of cytokines and on activation of activator protein-1 in RBL-2H3 cells. Phytomedicine. 2008; 15:177–184. PMID: 17950587.
Article3. Huang F, Yamaki K, Tong X, Fu L, Zhang R, Cai Y, Yanagisawa R, Inoue K, Takano H, Yoshino S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int Immunopharmacol. 2008; 8:502–507. PMID: 18279805.
Article4. Chang GT, Kang SK, Kim JH, Chung KH, Chang YC, Kim CH. Inhibitory effect of the Korean herbal medicine, Dae-Jo-Whan, on platelet-activating factor-induced platelet aggregation. J Ethnopharmacol. 2005; 102:430–439. PMID: 16125889.
Article5. Hancke JL, Burgos RA, Ahumada F. Schisandra chinensis (Turcz.) Baill. Fitoterapia. 1999; 70:451–471.6. Greene LS. Asthma, oxidant stress, and diet. Nutrition. 1999; 15:899–907. PMID: 10575668.
Article7. Shon MY, Choi SD, Kahng GG, Nam SH, Sung NJ. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004; 42:659–666. PMID: 15019191.
Article8. Ryu JS, Hyun JW, Kim HW, Shim MJ, Kim BK. Effects of artificial digestive juice on the antitumor-immunity activity of protein-bound polysaccharide from Ganoderma lucidum. Yakhak Hoeji. 2000; 44:347–353.9. Folin O, Denis W. A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J Biol Chem. 1915; 22:305–308.
Article10. Mărghitaş LA, Stanciu OG, Dezmirean DS, Bobiş O, Popescu O, Bogdanov S, Campos MG. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009; 115:878–883.
Article11. Choi HJ, Chung MJ, Ham SS. Antiobese and hypocholesterolaemic effects of an Adenophora triphylla extract in HepG2 cells and high fat diet-induced obese mice. Food Chem. 2010; 119:437–444.12. Yamashita K, Suzuki Y, Matsui T, Yoshimaru T, Yamaki M, Suzuki-Karasaki M, Hayakawa S, Shimizu K. Epigallocatechin gallate inhibits histamine release from rat basophilic leukemia (RBL-2H3) cells: role of tyrosine phosphorylation pathway. Biochem Biophys Res Commun. 2000; 274:603–608. PMID: 10924324.
Article13. Watanabe J, Shinmoto H, Tsushida T. Coumarin and flavone derivatives from estragon and thyme as inhibitors of chemical mediator release from RBL-2H3 Cells. Biosci Biotechnol Biochem. 2005; 69:1–6. PMID: 15665459.
Article14. Fukao T, Terauchi Y, Kadowaki T, Koyasu S. Role of phosphoinositide 3-kinase signaling in mast cells: new insights from knockout mouse studies. J Mol Med (Berl). 2003; 81:524–535. PMID: 12928787.
Article15. Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002; 56:276–282. PMID: 12224598.
Article16. Zhao Z, Egashira Y, Sanada H. Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J Nutr. 2004; 134:3083–3088. PMID: 15514279.
Article17. Lafay S, Gil-Izquierdo A, Manach C, Morand C, Besson C, Scalbert A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J Nutr. 2006; 136:1192–1197. PMID: 16614403.
Article18. Aura AM. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem Rev. 2008; 7:407–429.
Article19. Nishikawa H, Kitani S. Tea catechins have dual effect on mast cell degranulation induced by compound 48/80. Int Immunopharmacol. 2008; 8:1207–1215. PMID: 18602066.
Article20. Ramalakshmi K, Jagan Mohan Rao L, Takano-Ishikawa Y, Goto M. Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 2009; 115:79–85.
Article21. Wijngaard HH, Rössle C, Brunton N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009; 116:202–207.
Article22. Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, Chung HG, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol. 2007; 7:1678–1684. PMID: 17996677.
Article23. Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991; 254:707–710. PMID: 1948049.
Article24. Hines C. The diverse effects of mast cell mediators. Clin Rev Allergy Immunol. 2002; 22:149–160. PMID: 11975420.
Article25. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263. PMID: 9856950.
Article26. Yang G, Li L, Volk A, Emmell E, Petley T, Giles-Komar J, Rafferty P, Lakshminarayanan M, Griswold DE, Bugelski PJ, Das AM. Therapeutic dosing with anti-interleukin-13 monoclonal antibody inhibits asthma progression in mice. J Pharmacol Exp Ther. 2005; 313:8–15. PMID: 15644434.
Article27. Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990; 346:274–276. PMID: 2374592.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Oxidative and Anti-Inflammatory Effect of Water Extract from Perillae semen in RBL-2H3 Cells
- Inhibition of Interleukin-4 and β-Hexosaminidase Release in RBL-2H3 Cells by Compounds Isolated from Lobelia chinensis
- Imidacloprid inhibits IgE-mediated RBL-2H3 cell degranulation and passive cutaneous anaphylaxis
- Inhibitory effect of the aqueous extract of a tetraploid ‘etteum’ variety in Platycodon grandiflorum on degranulation and inflammatory mediator release in RBL-2H3 cells
- A Synthetic Chenodeoxycholic Acid Derivative, HS-1200-induced Apoptosis of RBL-2H3 Cells