Asia Pac Allergy.
2016 Oct;6(4):236-244. 10.5415/apallergy.2016.6.4.236.
Imidacloprid inhibits IgE-mediated RBL-2H3 cell degranulation and passive cutaneous anaphylaxis
- Affiliations
-
- 1State Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China. chenhongbing@ncu.edu.cn
- 2School of Food Science, Nanchang University, Nanchang 330047, China.
- 3Department of Rehabilitation, The First Affiliated Hospital of Nanchang University, Nanchang 330006, China.
- 4Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, China.
- KMID: 2397020
- DOI: http://doi.org/10.5415/apallergy.2016.6.4.236
Abstract
- BACKGROUND
Imidacloprid has been commonly used as a pesticide for crop protection and acts as nicotinic acetylcholine receptor agonists. Little information about the relationship between imidacloprid and allergy is available.
OBJECTIVE
This study aims to examine the effects of imidacoprid on IgE-mediated mast cell activation.
METHODS
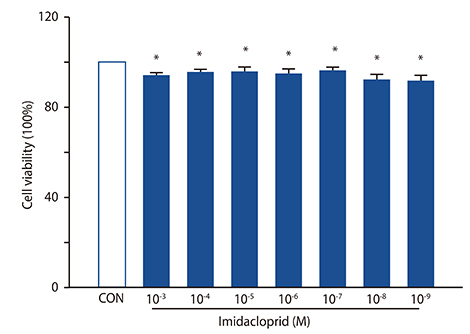
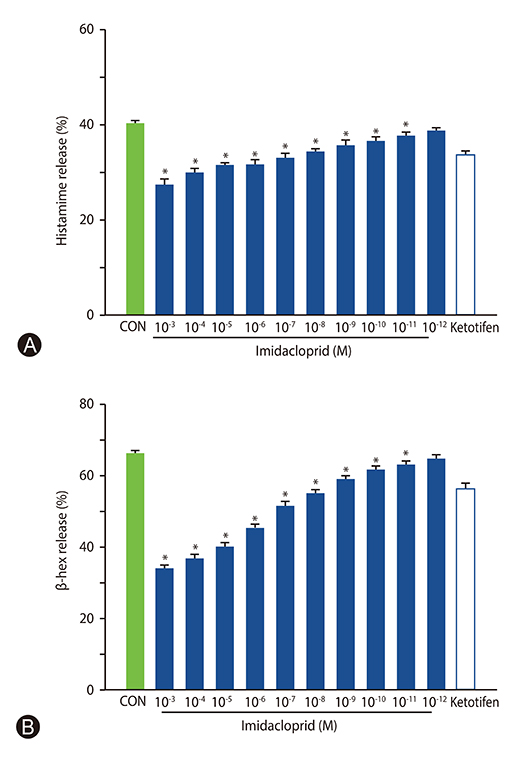
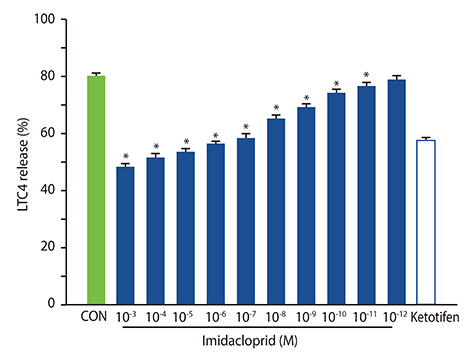
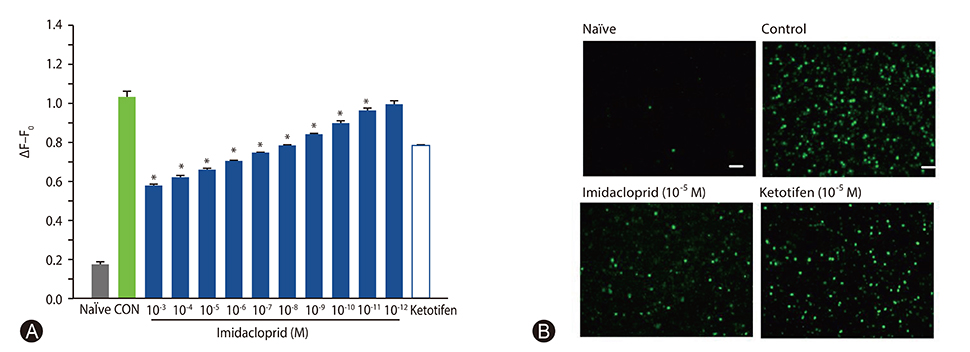
The rat basophilic leukemia cell line RBL-2H3 (RBL-2H3 cells) were treated with 10⻳- 10⻹² mol/L imidacloprid, followed by measuring the mediator production, influx of Ca²âº in IgE-activated RBL-2H3 cells, and the possible effects of imidacoprid on anti-dinitrophenyl IgE-induced passive cutaneous anaphylaxis (PCA).
RESULTS
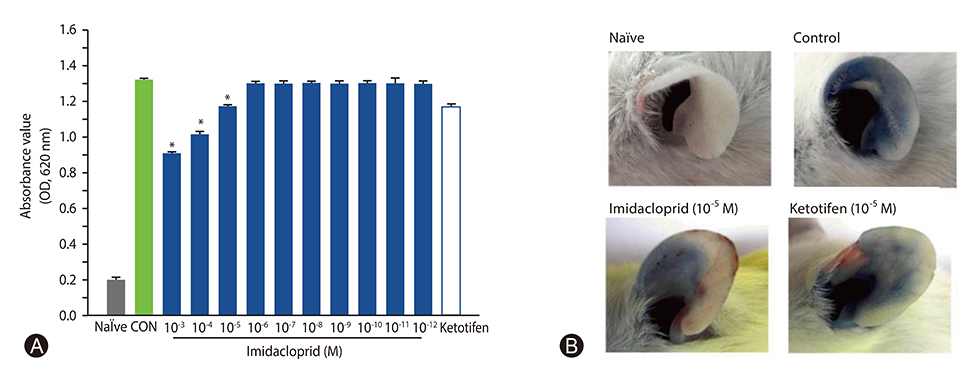
It was shown that imidacoprid suppressed the production of histamine, β-hexosaminidase, leukotriene C4, interleukin-6, tumor necrosis factor-α, and Ca²âº mobilization in IgE-activated RBL-2H3 cells and decreased vascular extravasation in IgE-induced PCA.
CONCLUSION
It is the first time to show that imidacloprid suppressed the activation of RBL-2H3 cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000; 173:131–140.
Article2. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005; 23:749–786.
Article3. Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008; 8:478–486.
Article4. Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001; 194:F1–F5.
Article5. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012; 18:693–704.
Article6. Schwartz LB. Mast cells: function and contents. Curr Opin Immunol. 1994; 6:91–97.
Article7. Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999; 402:6760 Suppl. B24–B30.8. Burr ML, Wat D, Evans C, Dunstan FD, Doull IJ. British Thoracic Society Research Committee. Asthma prevalence in 1973, 1988 and 2003. Thorax. 2006; 61:296–299.
Article9. Geraldini M, Rosario NA, Riedi CA, Leopoldino BB, Rosario CS, Barkema F, Palermo F, Macedo G, KusanoL LD, Eiras NO, Robl R, Schnekenberg RP, Ribeiro TB, Macedo V. Time Trends in the prevalence of allergic diseases in childhood. J Allergy Clin Immunol. 2010; 125:2 Suppl 1. AB31.
Article10. Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Immunol. 1999; 103(1 Pt 1):1–10.
Article11. von Mutius E. The rising trends in asthma and allergic disease. Clin Exp Allergy. 1998; 28:Suppl 5. 45–49.
Article12. Crinnion WJ. Do environmental toxicants contribute to allergy and asthma? Altern Med Rev. 2012; 17:6–18.13. Maizels RM. Infections and allergy - helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005; 17:656–661.
Article14. Yazdanbakhsh M, Wahyuni S. The role of helminth infections in protection from atopic disorders. Curr Opin Allergy Clin Immunol. 2005; 5:386–391.
Article15. Elbert A, Nauen R, Leicht W. Imidacloprid a novel chloronicotinyl insecticide: biological activity and agricultural importance.In : Ishaaya I, Degheele D, editors. Insecticides with novel modes of action: mechanisms and application. New York: Springer;1998. p. 50–73.16. Gawade L, Dadarkar SS, Husain R, Gatne M. A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem Toxicol. 2013; 51:61–70.
Article17. Tasei JN, Lerin J, Ripault G. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manage Sci. 2000; 56:784–788.18. Matsuda K, Shimomura M, Ihara M, Akamatsu M, Sattelle DB. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: electrophysiology, molecular biology, and receptor modeling studies. Biosci Biotechnol Biochem. 2005; 69:1442–1452.
Article19. Bhardwaj S, Srivastava MK, Kapoor U, Srivastava LP. A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem Toxicol. 2010; 48:1185–1190.
Article20. Demsia G, Vlastos D, Goumenou M, Matthopoulos DP. Assessment of the genotoxicity of imidacloprid and metalaxyl in cultured human lymphocytes and rat bone-marrow. Mutat Res. 2007; 634:32–39.
Article21. Kapoor U, Srivastava MK, Srivastava LP. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol. 2011; 49:3086–3089.
Article22. Mohany M, El-Feki M, Refaat I, Garraud O, Badr G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J Toxicol Sci. 2012; 37:1–11.
Article23. Badgujar PC, Jain SK, Singh A, Punia JS, Gupta RP, Chandratre GA. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ Toxicol Pharmacol. 2013; 35:408–418.
Article24. Gatne MM, Bhoir PS, Deore MD. Immunotoxicity studies of imidacloprid in rats. Toxicol Int. 2006; 13:89–92.25. Zhang NN, Park DK, Park HJ. The inhibitory activity of atractylenolide Ш, a sesquiterpenoid, on IgE-mediated mast cell activation and passive cutaneous anaphylaxis (PCA). J Ethnopharmacol. 2013; 145:278–285.
Article26. Ortega E, Hazan B, Zor U, Pecht I. Mast cell stimulation by monoclonal antibodies specific for the Fc epsilon receptor yields distinct responses of arachidonic acid and leukotriene C4 secretion. Eur J Immunol. 1989; 19:2251–2256.27. Yoshimaru T, Suzuki Y, Matsui T, Yamashita K, Ochiai T, Yamaki M, Shimizu K. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin Exp Allergy. 2002; 32:612–618.
Article28. Joo HM, Nam SY, Yang KH, Kim CS, Jin YW, Kim JY. The effects of low-dose ionizing radiation in the activated rat basophilic leukemia (RBL-2H3) mast cells. J Biol Chem. 2012; 287:27789–27795.
Article29. Molfetta R, Gasparrini F, Peruzzi G, Vian L, Piccoli M, Frati L, Santoni A, Paolini R. Lipid raft-dependent FcepsilonRI ubiquitination regulates receptor endocytosis through the action of ubiquitin binding adaptors. PLoS One. 2009; 4:e5604.30. Yao JH, Cui M, Li MT, Liu YN, He QH, Xiao JJ, Bai Y. Angiopoietin1 inhibits mast cell activation and protects against anaphylaxis. PLoS One. 2014; 9:e89148.
Article31. Han SY, Bae JY, Park SH, Kim YH, Park JH, Kang YH. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J Nutr. 2013; 143:632–639.
Article32. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993; 328:257–265.
Article33. Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004; 431:1007–1011.34. Fukuyama T, Kosaka T, Tajima Y, Ueda H, Hayashi K, Shutoh Y, Harada T. Prior exposure to organophosphorus and organochlorine pesticides increases the allergic potential of environmental chemical allergens in a local lymph node assay. Toxicol Lett. 2010; 199:347–356.
Article35. Nishino R, Fukuyama T, Tajima Y, Miyashita L, Watanabe Y, Ueda H, Kosaka T. Prior oral exposure to environmental immunosuppressive chemicals methoxychlor, parathion, or piperonyl butoxide aggravates allergic airway inflammation in NC/Nga mice. Toxicology. 2013; 309:1–8.
Article36. Narita S, Goldblum RM, Watson CS, Brooks EG, Estes DM, Curran EM, Midoro-Horiuti T. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ Health Perspect. 2007; 115:48–52.
Article37. Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, Tager IB. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006; 114:1916–1922.
Article38. Karmaus W, Kuehr J, Kruse H. Infections and atopic disorders in childhood and organochlorine exposure. Arch Environ Health. 2001; 56:485–492.
Article39. Reichrtová E, Ciznár P, Prachar V, Palkovicová L, Veningerová M. Cord serum immunoglobulin E related to the environmental contamination of human placentas with organochlorine compounds. Environ Health Perspect. 1999; 107:895–899.
Article40. European Food Safety Authority. Modification of the existing MRLs for imidacloprid in rice. EFSA J. 2010; 8:1589.41. Boyd RT. The molecular biology of neuronal nicotinic acetylcholine receptors. Crit Rev Toxicol. 1997; 27:299–318.
Article42. Kageyama-Yahara N, Suehiro Y, Yamamoto T, Kadowaki M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 2008; 377:321–325.
Article43. Kindt F, Wiegand S, Niemeier V, Kupfer J, Löser C, Nilles M, Kurzen H, Kummer W, Gieler U, Haberberger RV. Reduced expression of nicotinic alpha subunits 3, 7, 9 and 10 in lesional and nonlesional atopic dermatitis skin but enhanced expression of alpha subunits 3 and 5 in mast cells. Br J Dermatol. 2008; 159:847–857.44. Mishra NC, Rir-sima-ah J, Boyd RT, Singh SP, Gundavarapu S, Langley RJ, Razani-Boroujerdi S, Sopori ML. Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha 7/alpha 9/alpha 10-nicotinic receptors. J Immunol. 2010; 185:588–596.45. Yamamoto T, Kodama T, Lee J, Utsunomiya N, Hayashi S, Sakamoto H, Kuramoto H, Kadowaki M. Anti-allergic role of cholinergic neuronal pathway via α7 nicotinic ACh receptors on mucosal mast cells in a murine food allergy model. PLoS One. 2014; 9:e85888.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tryptophan Negatively Regulates IgE-mediated Mast Cell Activation
- Anti-Oxidative and Anti-Inflammatory Effect of Water Extract from Perillae semen in RBL-2H3 Cells
- Identification of Functionally Different Rat IgE in RBL-2H3 Exocytosis
- Inhibitory effects of epigallocatechin gallate on compound 48/80-inducedmast cell activation and passive cutaneous anaphylaxis
- Inhibitory effect of the aqueous extract of a tetraploid ‘etteum’ variety in Platycodon grandiflorum on degranulation and inflammatory mediator release in RBL-2H3 cells