Nutr Res Pract.
2010 Aug;4(4):311-316.
Birth weight was negatively correlated with plasma ghrelin, insulin resistance, and coenzyme Q10 levels in overweight children
- Affiliations
-
- 1Department of Food & Nutrition, Kyungnam University, 449 Woryeong-dong, Masanhappo-gu, Changwon-si, Gyeongnam 631-701, Korea. pej@kyungnam.ac.kr
Abstract
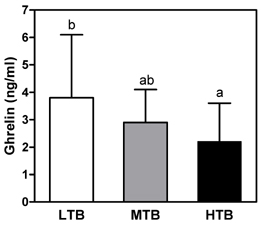
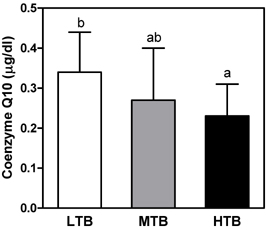
- The purpose of this study was to investigate the relationship between birth weight and appetite related hormones, insulin resistance, and antioxidant status in overweight children aged 9-10 years. Thirty-four healthy overweight children (18 boys, 16 girls) were evaluated with respect to anthropometric measurement, lipid profiles, leptin, ghrelin, glucose, insulin, C-peptide, lipid soluble vitamins, and antioxidant enzyme activities. I found that birth weight was negatively correlated with insulin resistance parameters, ghrelin, and coenzyme Q10 levels. There was a significant positive correlation between present BMI and leptin level, while a negative correlation was noted between the BMI and alpha-tocopherol and lycopene levels. When total subjects were classified into three groups by tertiles of birth weight, the lowest tertile of birth weight (LTB) group showed higher levels of fasting glucose, HOMA-IR, and ghrelin level than the highest tertile of birth weight (HTB) groups. On the other hand, HTB group showed an increased oxidative stress (decreased coenzyme Q10 level and catalase activity) compared to the LTB group. In conclusion, plasma ghrelin level might play an important role in accelerated growth in overweight children with LTB. Increased insulin resistance is present in overweight children with LTB, while decreased coenzyme Q10 and catalase activity in overweight children with HTB. These results suggest that birth weight might be an important factor for determination of treatment for obesity related complications in childhood obesity.
MeSH Terms
-
Aged
alpha-Tocopherol
Appetite
Birth Weight
C-Peptide
Carotenoids
Catalase
Child
Fasting
Ghrelin
Glucose
Hand
Humans
Insulin
Insulin Resistance
Leptin
Obesity
Overweight
Oxidative Stress
Parturition
Plasma
Salicylates
Ubiquinone
Vitamins
C-Peptide
Carotenoids
Catalase
Ghrelin
Glucose
Insulin
Leptin
Salicylates
Ubiquinone
Vitamins
alpha-Tocopherol
Figure
Reference
-
1. Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Curr Opin Endocrinol Diabetes Obes. 2008. 15:21–29.
Article2. Kim HM, Park J, Kim HS, Kim DH, Park SH. Obesity and cardiovascular risk factors in Korean children and adolescents aged 10-18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am J Epidemiol. 2006. 164:787–793.
Article3. Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006. 65:65–69.
Article4. Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young adult men--a prospective twin study. Int J Obes Relat Metab Disord. 2001. 25:1537–1545.
Article5. Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young women: a prospective twin study. Am J Clin Nutr. 2002. 75:676–682.
Article6. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.
Article7. Steinberger J, Daniels SR. American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young). American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation. 2003. 107:1448–1453.
Article8. Barker DJ. The fetal origins of coronary heart disease. Eur Heart J. 1997. 18:883–884.
Article9. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005. 115:e290–e296.
Article10. Chiavaroli V, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics. 2009. 124:695–702.
Article11. Mohn A, Chiavaroli V, Cerruto M, Blasetti A, Giannini C, Bucciarelli T, Chiarelli F. Increased oxidative stress in prepubertal children born small for gestational age. J Clin Endocrinol Metab. 2007. 92:1372–1378.
Article12. Hillestrøm PR, Weimann A, Jensen CB, Storgaard H, Vaag AA, Poulsen HE. Consequences of low birthweight on urinary excretion of DNA markers of oxidative stress in young men. Scand J Clin Lab Invest. 2006. 66:363–370.
Article13. Darendeliler F, Bas F, Bundak R, Coban A, Disci R, Sancakli O, Gokcay G, Ince Z, Can G. Elevated ghrelin levels in preterm born children during prepubertal ages and relationship with catch-up growth. Eur J Endocrinol. 2008. 159:555–560.
Article14. Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005. 135:1320–1325.
Article15. Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006. 89:71–84.
Article16. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001. 50:707–709.
Article17. Rosická M, Krsek M, Matoulek M, Jarkovská Z, Marek J, Justová V, Lacinová Z. Serum ghrelin levels in obese patients: the relationship to serum leptin levels and soluble leptin receptors levels. Physiol Res. 2003. 52:61–66.18. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002. 87:240–244.
Article19. Himes JH, Dietz WH. The Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. Am J Clin Nutr. 1994. 59:307–316.
Article20. Committee on Health and Statistics, Korean Pediatrics Society. 1998 Korean physical growth standards for children and adolescents. 1999. Seoul: Guang Mun Publishing Company.21. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000. 284:3043–3045.22. Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.
Article23. Jakob E, Elmadfa I. Rapid HPLC-Assay for assessment of vitaminK1, A, E and beta-carotene status in children (7-19 years). Int J Vitam Nutr Res. 1995. 65:31–35.24. Beutler E. Red cell metabolism: A mannual for biochemical methods. 1984. New York: Verlag Grune and Stratton;71–73.25. Aebi H. Methods of enzymatic analysis. 1974. Weinheim: Verlag Chemie GmbH;673–684.26. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article27. Thurnham DI, Davis JA, Crump BJ, Situnayake RD, Davis M. The use ofdifferent lipids to express serum tocopherol/lipid ratios for themeasurement of vitamin E status. Ann Clin Biochem. 1986. 23:514–520.
Article28. Horwitt MK, Harvey CC, Dahm CH Jr, Searcy MT. Relationship between tocopherol and serum lipid levels for determination of nutritional adequacy. Ann N Y Acad Sci. 1972. 203:223–236.
Article29. Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschöp M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001. 145:669–673.
Article30. McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004. 89:1630–1635.
Article31. Lee JJ, Park CG, Lee KS. Birth weight distribution by gestational age in Korea population: Using finite mixture model. Korean Journal of Pediatrics. 2005. 48:1179–1186.32. Cortelazzi D, Cappiello V, Morpurgo PS, Ronzoni S, Nobile De Santis MS, Cetin I, Beck-Peccoz P, Spada A. Circulating levels of ghrelin in human fetuses. Eur J Endocrinol. 2003. 149:111–116.
Article33. Bellone S, Rapa A, Vivenza D, Castellino N, Petri A, Bellone J, Me E, Broglio F, Prodam F, Ghigo E, Bona G. Circulating ghrelin levels as function of gender, pubertal status and adiposity in childhood. J Endocrinol Invest. 2002. 25:RC13–RC15.
Article34. Iñiguez G, Ong K, Peña V, Avila A, Dunger D, Mericq V. Fasting and post-glucose ghrelin levels in SGA infants: relationships with size and weight gain at one year of age. J Clin Endocrinol Metab. 2002. 87:5830–5833.
Article35. Méndez-Ramírez F, Barbosa-Sabanero G, Romero-Gutiérrez G, Malacara JM. Ghrelin in small-for-gestational age (SGA) newborn babies: a cross-sectional study. Clin Endocrinol (Oxf). 2009. 70:41–46.
Article36. Eyzaguirre F, Mericq V. Insulin resistance markers in children. Horm Res. 2009. 71:65–74.
Article37. Dabalea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999. 22:944–950.
Article38. Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001. 86:267–272.
Article39. Vaag A, Jensen CB, Poulsen P, Brøns C, Pilgaard K, Grunnet L, Vielwerth S, Alibegovic A. Metabolic aspects of insulin resistance in individuals born small for gestational age. Horm Res. 2006. 65:137–143.
Article40. Bouhours-Nouet N, Dufresne S, de Casson FB, Mathieu E, Douay O, Gatelais F, Rouleau S, Coutant R. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects. Diabetes Care. 2008. 31:1031–1036.
Article41. Brufani C, Grossi A, Fintini D, Tozzi A, Nocerino V, Patera PI, Ubertini G, Porzio O, Barbetti F, Cappa M. Obese children with low birth weight demonstrate impaired beta-cell function during oral glucose tolerance test. J Clin Endocrinol Metab. 2009. 94:4448–4452.
Article42. Costa GB, Horta N, Resende ZF, Souza G, Barreto LM, Correia LH, Nascimento TA, Rios CB, Barreto-Filho JA, Lopes HF. Body mass index has a good correlation with proatherosclerotic profile in children and adolescents. Arq Bras Cardiol. 2009. 93:261–267.43. d'Annunzio G, Vanelli M, Pistorio A, Minuto N, Bergamino L, Lafusco D, Lorini R. Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Insulin resistance and secretion indexes in healthy Italian children and adolescents: a multicentre study. Acta Biomed. 2009. 80:21–28.44. Strauss RS. Comparison of serum concentration of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and non obese children. J Pediatr. 1999. 134:160–165.
Article45. Reitman A, Friedrich I, Ben-Amotz A, Levy Y. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity. Isr Med Assoc J. 2002. 4:590–593.46. Saker M, Soulimane Mokhtari N, Merzouk SA, Merzouk H, Belarbi B, Narce M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur J Obstet Gynecol Reprod Biol. 2008. 141:95–99.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma Ghrelin Levels and Its Relationship with Obesity in Obese Children
- Correlation between Plasma Ghrelin Levels and Risperidone Induced Weight Gain
- Serum Concentrations of Ghrelin and Leptin according to Thyroid Hormone Condition, and Their Correlations with Insulin Resistance
- Relationship between Serum Ghrelin and Insulin Resistance in Obese Children and Adolescents
- Association of Plasma Ghrelin Levels with Insulin Resistance in Type 2 Diabetes Mellitus among Saudi Subjects