Nutr Res Pract.
2008 Mar;2(1):17-21.
Plasma, tissue and urinary levels of aloin in rats after the administration of pure aloin
- Affiliations
-
- 1Department of Food and Nutrition, Sookmyung Women's University, Seoul 140-742, Korea. mksung@sm.ac.kr
- 2Department of Food and Nutrition, Seoul National University, Seoul 151-742, Korea.
Abstract
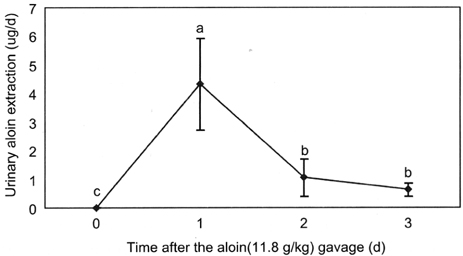
- Aloin is a physiologically active anthraquinone present in aloe. There are two isomers of aloin, aloin A and aloin B, occurring as a mixture of diastereomers. The objective of this study was to determine the bioavailability and tissue distribution of aloin. Rats were gavaged with 11.8g/kg aloin, and the levels of aloin and its conjugates were measured in plasma, tissues, and urine. Plasma aloin level showed a peak at 1hr after the administration and the concentration was 59.07+/-10.5 ng/ml. The 24 h cumulated urinary aloin was 0.03% of the initial dose. These results suggest that aloin is absorbed and reaches a peak plasma level within 1-1.5 h after the administration and a significant portion is possibly metabolized or is excreted in feces. These results can apply to the determination of the adequate intake level of aloe and aloe products to achieve the desired biological effect, and to interprete in vitro study results.
Figure
Reference
-
1. Avila H, Rivero J, Herrera F, Fraile G. Cytotoxicity of a low molecular weight fraction from Aloe vera (Aloe barbadensis Miller) gel. Toxicon. 1997. 35:1423–1430.
Article2. Bolkent S, Akev N, Ozsoy N, Sengezer-Inceli M, Can A, Alper O, Yanardag R. Effect of Aloe vera (L.) Burm. Fil. Leaf gel and pulp extracts on kidney in type-II diabetic rat models. Indian J Exp Biol. 2004. 42:48–52.3. Bub A, Watzlm B, Heeb D, Rechkemmer G, Briviba K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur J Nutr. 2001. 40:113–120.
Article4. Can A, Akev N, Ozsoy N, Bolkent S, Arda BP, Yanardag R, Okyar A. Effect of Aloe vera leaf gel and pulp eatracts on the liver in type-II diabetic rat models. Biol Pharm Bull. 2004. 27:694–698.
Article5. Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997. 25:1045–1050.6. Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001. 10:53–58.7. Chung JH, Cheong JC, Lee JY, Roh HK, Cha YN. Acceleration of the alcohol oxidation rate in rats with aloin, a quinine derivative of Aloe. Biochem Pharmacol. 1996. 52:1461–1468.
Article8. Davis RH, DiDonato JJ, Johnson RW, Stewart CB. Aloe vera, hydrocortisone, and sterol influence on wound tensile strength and anti-inflammation. J Am Podiatr Med Assoc. 1994. 84:614–621.
Article9. Day AJ, Cañada FJ, Díaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000. 25:166–170.
Article10. Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998. 25:71–75.11. Esmat AY, El-Gerzawy SM, Rafaat A. DNA ploidy and S phase fraction of breast and ovarian tumor cells treated with a natural anthracycline analog (aloin). Cancer Biol Ther. 2005. 4:108–112.
Article12. Esmat AY, Tomasetto C, Rio MC. Cytotoxicity of a natural anthraquinone (aloin) against human breast cancer cell lines with and without ErbB-2: topoisomerase II alpha coamplification. Cancer Biol Ther. 2006. 5:97–103.
Article13. Groon QJ, Reynols T. Barbaloin in aloe species. Planta Med. 1987. 53:345–348.14. Jäger W, Zembsch B, Wolschann P, Pittenauer E, Senderowicz AM, Sausville EA, Sedlacek HH, Graf J, Thalhammer T. Metabolism of the anticancer drug flavopiridol, a new inhibitor of cyclin dependent kinases, in rat liver. Life Sci. 1998. 62:1861–1873.
Article15. Kim SH, Park HJ, Lee CM, Choi IW, Moon DO, Roh HJ, Lee HK, Park YM. Epigallocatechin-3-gallate protects toluene diisocyanate-induced airway inflammation in a murine model of asthma. FEBS Lett. 2006. 580:1883–1890.
Article16. Koch A. Metabolism of aloin-the influence of nutrition. J Pharm Biomed Anal. 1996. 14:1335–1338.17. Korkina L, Suprun M, Petrova A, Mikhal'chik E, Luci A, De Luca C. The protective and healing effects of a natural antioxidant formulation based on ubiquinol and Aloe vera against dextran sulfate-induced ulcerative colitis in rats. Biofactors. 2003. 18:255–264.
Article18. Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A novel prodrug of the green tea polyphenol(-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007. 67:4303–4310.
Article19. Maity TK, Mandal SC, Bhakta T, Pal M, Saha BP. Metabolism of 1,8-dihydroxy 3-hydroxy methyl anthraquinone (aloe-emodin) isolated from the leaves of Cassia tora in albino rats. Phytother Res. 2001. 15:459–460.
Article20. Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J Agric Food Chem. 2001. 49:1546–1551.
Article21. Mueller SO, Stopper H, Dekant W. Biotransformation of the anthraquinones emodin and chrysophanol by cytochrome P450 enzymes. Bioactivation to genotoxic metabolites. Drug Metab Dispos. 1998. 26:540–546.22. O'Leary KA, Day AJ, Needs PW, Mellon FA, O'Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003. 65:479–491.23. Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4'-glucoside do not differ in humans. J Nutr. 2000. 130:1200–1203.
Article24. Park YG, Park MY, Sung MK, Kwon H. Study on the intake pattern of health intended foods depending on inclusion of proclaimed health functional food materials. Journal of the Korean Society of Food Science and Nutrition. 2005. 34:374–379.
Article25. Reynold T. The compounds in Aloe leaf exudates: a review. Bot J Lin Soc. 1985. 90:157–177.26. Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995. 22:375–383.
Article27. Shimpo K, Chihara T, Beppu H, Ida C, Kaneko T, Hoshino M, Kuzuya H. Inhibition of azoxymethane-induced DNA adduct formation by Aloe arborescens var. natalensis. Asian Pac J Cancer Prev. 2003. 4:247–251.28. Tom R. Aloes-the genus aloe. 2004. Florida. USA: CRC press;40–47.29. Walle UK, Galijatovic A, Walle T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol. 1999. 58:431–438.
Article30. Wamer WG, Vath P, Falvey DE. In vitro studies on the photobiological properties of aloe emodin and aloin A. Free Radic Biol Med. 2003. 34:233–242.
Article31. Wolffram S, Block M, Ader P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J Nutr. 2002. 132:630–635.
Article32. Zhou Y, Feng Y, Wang H, Yang H. 90-day subchronic toxicity study of aloe whole-leaf powder. Wei Sheng Yan Jiu. 2003. 32:590–593.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anthraquinone Glycoside Aloin Induces Osteogenic Initiation of MC3T3-E1 Cells: Involvement of MAPK Mediated Wnt and Bmp Signaling
- Intestinal absorption of aloin, aloe-emodin, and aloesin; A comparative study using two in vitro absorption models
- Single- and repeated-dose toxicities of aloe fermentation products in rats
- Effect of administration of etretinate and fish oil on plasma cholesterol levels in rats
- In Vitro Effects of Plasma Collected From Rats Administered Naftopidil on Whole Urinary Bladder Preparation Isolated From Rats