Nutr Res Pract.
2009 Mar;3(1):9-14.
Intestinal absorption of aloin, aloe-emodin, and aloesin; A comparative study using two in vitro absorption models
- Affiliations
-
- 1Department of Food and Nutrition, Sookmyung Women's University, 52 Hyochangwon-gil, Yonsan-gu, Seoul 140-742, Korea. mksung@sm.ac.kr
- 2Department of Food and Nutrition, Seoul National University, 599 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea.
Abstract
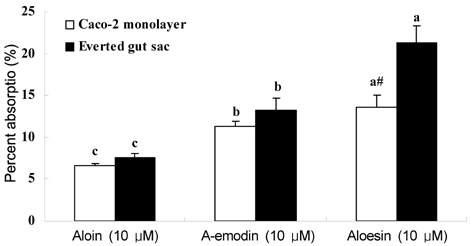
- Aloe products are one of the top selling health-functional foods in Korea, however the adequate level of intake to achieve desirable effects are not well understood. The objective of this study was to determine the intestinal uptake and metabolism of physiologically active aloe components using in vitro intestinal absorption model. The Caco-2 cell monolayer and the everted gut sac were incubated with 5-50 micrometer of aloin, aloe-emodin, and aloesin. The basolateral appearance of test compounds and their glucuronosyl or sulfated forms were quantified using HPLC. The % absorption of aloin, aloe-emodin, and aloesin was ranged from 5.51% to 6.60%, 6.60% to 11.32%, and 7.61% to 13.64%, respectively. Up to 18.15%, 18.18%, and 38.86% of aloin, aloe-emodin, and aloesin, respectively, was absorbed as glucuronidated or sulfated form. These results suggest that a significant amount is transformed during absorption. The absorption rate of test compounds except aloesin was similar in two models; more aloesin was absorbed in the everted gut sac than in the Caco-2 monolayer. These results provide information to establish adequate intake level of aloe supplements to maintain effective plasma level.
Keyword
MeSH Terms
Figure
Reference
-
1. Alves DS, Pérez-Fons L, Estepa A, Micol V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem Pharmacol. 2004. 68:549–561.
Article2. Azuma K, Ippoushi K, Ito H, Higashio H, Terao J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J Agric Food Chem. 2002. 50:1706–1712.
Article3. Barthe L, Woodley J, Houin G. Gastrointestinal absorption of drugs: methods and studies. Fundam Clin Pharmacol. 1999. 13:154–168.
Article4. Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK, Suri KA, Suri J, Bhadauria M, Singh B. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2007. 111:560–566.
Article5. Davis RH, Donato JJ, Hartman GM, Haas RC. Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J Am Podiatr Med Assoc. 1994. 84:77–81.
Article6. Esmat AY, Tomasetto C, Rio MC. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2: topoisomerase IIalpha coamplification. Cancer Biol Ther. 2006. 5:97–103.
Article7. Gee JM, DuPont MS, Rhodes MJ, Johnson IT. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic Biol Med. 1998. 25:19–25.
Article8. Hadjeri M, Barbier M, Ronot X, Mariotte AM, Boumendjel A, Boutonnat J. Modulation of P-glycoprotein-mediated multidrug resistance by flavonoid derivatives and analogues. J Med Chem. 2003. 46:2125–2131.
Article9. Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989. 96:736–749.
Article10. Ishii Y, Tanizawa H, Takino Y. Studies of aloe. V. Mechanism of cathartic effect. Biol Pharm Bull. 1994. 17:651–653.11. Kanai Y, Nakai Y, Nakajima N, Tanayama S. Metabolic disposition of 6-ethyl-3-(1H-tetrazol-5-yl)-chromone, a new antiallergic agent, in the rat, guinea-pig, rabbit, dog and monkey. Xenobiotica. 1979. 9:33–50.
Article12. Keller RP, Neville MC. Determination of total protein in human milk: comparison of methods. Clin Chem. 1986. 32:120–123.
Article13. Korkina L, Suprun M, Petrova A, Mikhal'chik E, Luci A, De Luca C. The protective and healing effects of a natural antioxidant formulation based on ubiquinol and aloe vera against dextran sulfate-induced ulcerative colitis in rats. Biofactors. 2003. 18:255–264.
Article14. Lambert N, Kroon PA, Faulds CB, Plumb GW, McLauchlan WR, Day AJ, Williamson G. Purification of cytosolic beta-glucosidase from pig liver and its reactivity towards flavonoid glycosides. Biochim Biophys Acta. 1999. 1435:110–116.
Article15. Liu X, Tam VH, Hu M. Disposition of flavonoids via enteric recycling: determination of the UDP-glucuronosyltransferase isoforms responsible for the metabolism of flavonoids in intact Caco-2 TC7 cells using siRNA. Mol Pharm. 2007. 4:873–882.
Article16. Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of aloe vera used for burn wound healing: a systematic review. Burns. 2007. 33:713–718.
Article17. Mailleau C, Capeau J, Brahimi-Horn MC. Interrelationship between the Na+/glucose cotransporter and CFTR in Caco-2 cells: relevance to cystic fibrosis. J Cell Physiol. 1998. 176:472–481.
Article18. Maurizis JC, Nicolas C, Verny M, Ollier M, Faurie M, Payard M, Veyre A. Biodistribution and metabolism in rats and mice of bucromarone. Drug Metab Dispos. 1991. 19:94–99.19. McCarthy TJ, Haynes LJ. The distribution of alosein in some south African aloe species. Planta Med. 1967. 15:342–344.
Article20. Miao H, Yang Z. Regiospecific carbonylative annulation of iodophenol acetates and acetylenes to construct the flavones by a new catalyst of palladium-thiourea-dppp complex. Org Lett. 2000. 2:1765–1768.
Article21. Moore Z, Cowman S. A systematic review of wound cleansing for pressure ulcers. J Clin Nurs. 2008. 17:1963–1972.
Article22. Muni IA, Leeling JL, Helms RJ, Johnson N. Antiallergic chromones. I. Disposition of 5-(3-p-cyanophenoxy-2-hydroxy-1-propoxy)-2-(1H-tetrazol-5-yl) chromone in four mammalian species. Toxicol Appl Pharmacol. 1978. 43:527–534.
Article23. Németh K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003. 42:29–42.24. Ni Y, Turner D, Yates K, Tizard I. Stabilization of growth factors relevant to wound healing by a plant cell wall biomaterial. Planta Med. 2007. 73:1260–1266.
Article25. Ollila F, Halling K, Vuorela P, Vuorela H, Slotte JP. Characterization of flavonoid--biomembrane interactions. Arch Biochem Biophys. 2002. 399:103–108.
Article26. Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4'-glucoside do not differ in humans. J Nutr. 2000. 130:1200–1203.
Article27. Paine MF, Fisher MB. Immunochemical identification of UGT isoforms in human small bowel and in caco-2 cell monolayers. Biochem Biophys Res Commun. 2000. 14:1053–1057.
Article28. Park YG, Park MY, Sung MK, Kwon H. Study on the intake pattern of health intended foods depending on inclusion of proclaimed health functional food materials. Journal of the Korean Society of Food Science and Nutrition. 2005. 34:374–379.
Article29. Saccù D, Bogoni P, Procida G. Aloe exudate: characterization by reversed phase HPLC and headspace GC-MS. J Agric Food Chem. 2001. 49:4526–4530.
Article30. Speranza G, Morelli CF, Tubaro A, Altinier G, Durì L, Manitto P. Aloeresin I, an anti-inflammatory 5-methylchromone from cape aloe. Planta Med. 2005. 71:79–81.
Article31. Tammela P, Laitinen L, Galkin A, Wennberg T, Heczko R, Vuorela H, Slotte JP, Vuorela P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch Biochem Biophys. 2004. 425:193–199.
Article32. Teng ZH, Zhou SY, Ran YH, Liu XY, Yang RT, Yang X, Yuan CJ, Mei QB. Cellular absorption of anthraquinones emodin and chrysophanol in human intestinal Caco-2 cells. Biosci Biotechnol Biochem. 2007. 71:1636–1643.
Article33. Vaidyanathan JB, Walle T. Glucuronidation and sulfation of the tea flavonoid (-)-epicatechin by the human and rat enzymes. Drug Metab Dispos. 2002. 30:897–903.
Article34. Vereczkey L, Jemnitz K, Monostory K, Veres Z, Kóbori L. The role of drug metabolizing enzymes in the effect and side-effect of the drugs. Orv Hetil. 2005. 146:947–952.35. Walgren RA, Lin JT, Kinne RK, Walle T. Cellular uptake of dietary flavonoid quercetin 4'-beta-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther. 2000. 294:837–843.36. Wilkinson AP, Gee JM, Dupont MS, Needs PW, Mellon FA, Williamson G, Johnson IT. Hydrolysis by lactase phlorizin hydrolase is the first step in the uptake of daidzein glucosides by rat small intestine in vitro. Xenobiotica. 2003. 33:255–264.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma, tissue and urinary levels of aloin in rats after the administration of pure aloin
- Single- and repeated-dose toxicities of aloe fermentation products in rats
- Aloe-Emodin Induces Chondrogenic Differentiation of ATDC5 Cells via MAP Kinases and BMP-2 Signaling Pathways
- Riboflavin and Thiamine Absorption
- Absorption and Half-Life