Nutr Res Pract.
2007 Jun;1(2):79-83.
Plasma total homocysteine and macrovascular complications are associated with food and nutrient intake in patients with Type II diabetes mellitus
- Affiliations
-
- 1Department of Nutritional Sciences, Ewha Womans University, Seoul 120-750, Korea. nschang@ewha.ac.kr
- 2Department of Internal Medicine, Pochon CHA University College of Medicine, Sungnam, Gyeonggi 463-836, Korea.
Abstract
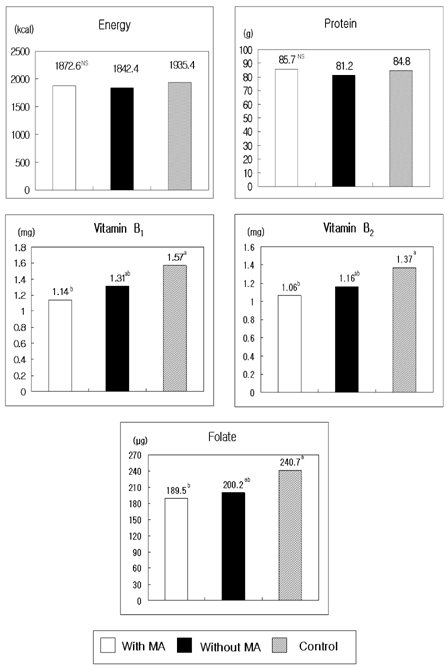
- The present study was conducted to document the association between plasma homocysteine levels and the presence of macrovascular angiopathy with food and nutrient intake patterns among patients with Type II diabetes mellitus in Korea. Plasma total homocysteine concentration was analyzed by HPLC-fluorescence detector method in 127 patients with non-insulin dependent diabetes mellitus. Logistic regression analyses were performed respectively to study the association of plasma homocysteine levels with clinical and dietary characteristics and macroangiopathy (MA). The average plasma homocysteine level of patients with MA was 14.2 micromol/l, which was significantly higher than that of patients without MA (11.4 micromol/l). The proportions of patients with MA showed a significant difference, being 32.3% in hyperhomocysteinemic (>14.0 micromol/l) patients and 13.5% in others with homocysteine levels lower than 14.0 micromol/l. Odds ratios for macroangiopathy by tertile increase of plasma homocysteine concentration were 1.633 (Q2) and 4.831 (Q3), when adjusted for age, sex, and cigarette smoking. Patients with MA consumed reduced amounts of vitamin B1, B2, and folate. The results indicate that the plasma homocysteine levels are significantly increased in NIDDM patients who have macroangiopathy. Dietary management such as increased fruits and vegetables and decreased potatoes and starches might be beneficial for the prevention of macroangiopathy in diabetic patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987. 422:43–52.
Article2. Araki A, Sako Y, Ito H. Plasma homocysteine concentrations in Japanese patients with non-insulin-dependent diabetes mellitus: effect of parenteral methylcobalamine treatment. Atherosclerosis. 1993. 103:149–157.
Article3. Bermejo LM, Aparicio A, Andrés P, López-Sobaler AM, Ortega RM. The influence of fruit and vegetable intake on the nutritional status and plasma homocysteine levels of institutionalised elderly people. Public Health Nutr. 2007. 10:266–272.
Article4. Berwanger CS, Jeremy JY, Stansby G. Homocysteine and vascular disease. Br J Surg. 1995. 82:726–731.
Article5. Bogers RP, Dagnelie PC, Bast A, van Leeuwen M, van Klaveren JD, van den Brandt PA. Effect of increased vegetable and fruit consumption on plasma folate and homocysteine concentrations. Nutrition. 2007. 23:97–102.
Article6. De Bree A, Verschuren WMM, Blom HJ, Kromhout D. Association between B vitamin intake and plasma homocysteine concentration in the general Dutch population aged 20-65y. Am J Clin Nutr. 2001. 73:1027–1033.
Article7. Hatzis CM, Bertsias GK, Linardakis M, Scott JM, Kafatos AG. Dietary and other lifestyle correlates of serum folate concentrations in a healthy adult population in Crete, Greece: a cross-sectional study. Nutr J. 2006. 5:5.
Article8. Hofmann MA, Kohl B, Zumbach MS, Borcea V, Bierhaus A, Henkels M, Amiral J, Schmidt AM, Fiehn W, Ziegler R, Wahl P, Nawroth PP. Hyperhomocysteinemia and endothelial dysfunction in IDDM. Diabetes Care. 1998. 2:841–848.
Article9. Hoogeveen EK, Kostense PJ, Beks PJ, Mackaay AJC, Jakobs C, Bouter LM, Heine RJ, Stehouwer CDA. Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin-dependent diabetes mellitus a population-based study. Arterioscler Thromb Vasc Biol. 1998. 18:133–138.
Article10. Hultberg B, Agardh E, Andersson A, Brattstrom L, Isaksson A, Israelsson B, Agardh CD. Increased levels of plasma homocysteine associated with nephropathy, but not severe retinopathy in type I diabetes mellitus. Scand J Clin Lab Invest. 1991. 51:277. [Abstract].
Article11. Jacques PF, Bostrom AG, Wilson PWF, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001. 73:613–621.
Article12. Korean Nutrition Society. Korean Dietary Reference Intakes. 2005. Seoul. Republic of Korea: Kookjin Publishing Co.13. Konstantinova SV, Vollset SE, Berstad P, Ueland PM, Drevon CA, Refsum H, Tell GS. Dietary predictors of plasma total homocysteine in the Hordaland Homocysteine Study. Br J Nutr. 2007. 98:201–210.
Article14. Masser PA, Taylor LM, Porter JM. Importance of elevated plasma homocysteine levels as a risk factor for atherosclerosis. Ann Thorac Surg. 1994. 58:1240–1246.
Article15. Munshi MN, Stone A, Fink L, Fonseca V. Hyperhomocysteinemia following a methionine load in patients with non-insulin-dependent diabetes mellitus and macrovascular disease. Metabolism. 1996. 45:133–135.
Article16. Park JY, Lee KU, Kim CH, Kim HK, Hong SK, Park KS, Lee HK, Min HK. Past and current obesity in Koreans with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1997. 35:49–56.
Article17. Paterson E, Gordon MH, Niwat C, George TW, Parr L, Waroonphan S, Lovegrove JA. Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter markers of oxidative stress or cardiovascular risk factors. J Nutr. 2006. 136:2849–2855.
Article18. Rees MM, Rodgers GM. Homocysteinemia: association of a metabolic disorder with vascular disease and thrombosis. Thromb Res. 1993. 71:337–359.
Article19. Robinson K, Mayer EL, Miller DP, Green R, van Lente F, Gupta A, Kottke-Marchant K, Savon SR, Selhub J, Nissen SE, Kutner M, Topol EJ, Jacobsen DW. Hyperhomocysteinemia and low pyridoxal phosphate common and independent reversible risk factors for coronary artery disease. Circulation. 1995. 92:2825–2830.20. Selhub J, Jacques PF, Wilson PWF, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993a. 270:2693–2698.
Article21. Selhub J, Jacques PF, Wilson PWF, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. J Am Med Assoc. 1993b. 272:2703–2708.
Article22. Stampfer MJ, Malinow MR. Can lowering homocysteine levels reduce cardiovascular risk? N Engl J Med. 1995. 332:328–329.
Article23. Tan PS, Wenlock RW, Buss DH. Folic acid content of the diet in various types of British household. Hum Nutr Appl Nutr. 1984. 38:17–22.24. Ubbink JB, Vermaak WJH, Bissbort S. Rapid high performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr. 1991. 565:441–446.
Article25. Ubbink JB. Vitamin nutrition status and homocysteine: an atherogenic risk factor. Nutr Rev. 1994. 52:383–393.
Article26. Vaccaro O, Ingrosso D, Rivellese A, Greco G, Riccardi G. Moderate hyperhomocysteinemia and retinopathy in insulin dependent diabetes. Lancet. 1997. 349:1102–1103.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Folic Acid and Ascorbate Supplementation on Plasma Homocysteine and Oxidative Stress in Patients with Type 2 Diabetes Mellitus
- The Relationship among Homocysteine, Bilirubin, and Diabetic Retinopathy
- Relationship between Serum Homocysteine Levels and Vascular Complications in Type 2 Diabetic Patients
- Relationship between plasma homocysteine levels and chronic diabetic complications in NIDDM patients
- Carbohydrate Consumption and Glycemic Index of the Usual Diet in Type 2 Diabetes Mellitus Patients