Lab Med Online.
2013 Oct;3(4):253-258.
Methods for Flow Cytometric Analysis of T Cell Subsets in HIV-infected Patients: 2-Color versus 4-Color
- Affiliations
-
- 1Department of Laboratory Medicine, Ajou University School of Medicine, Suwon, Korea. sungran@ajou.ac.kr
Abstract
- BACKGROUND
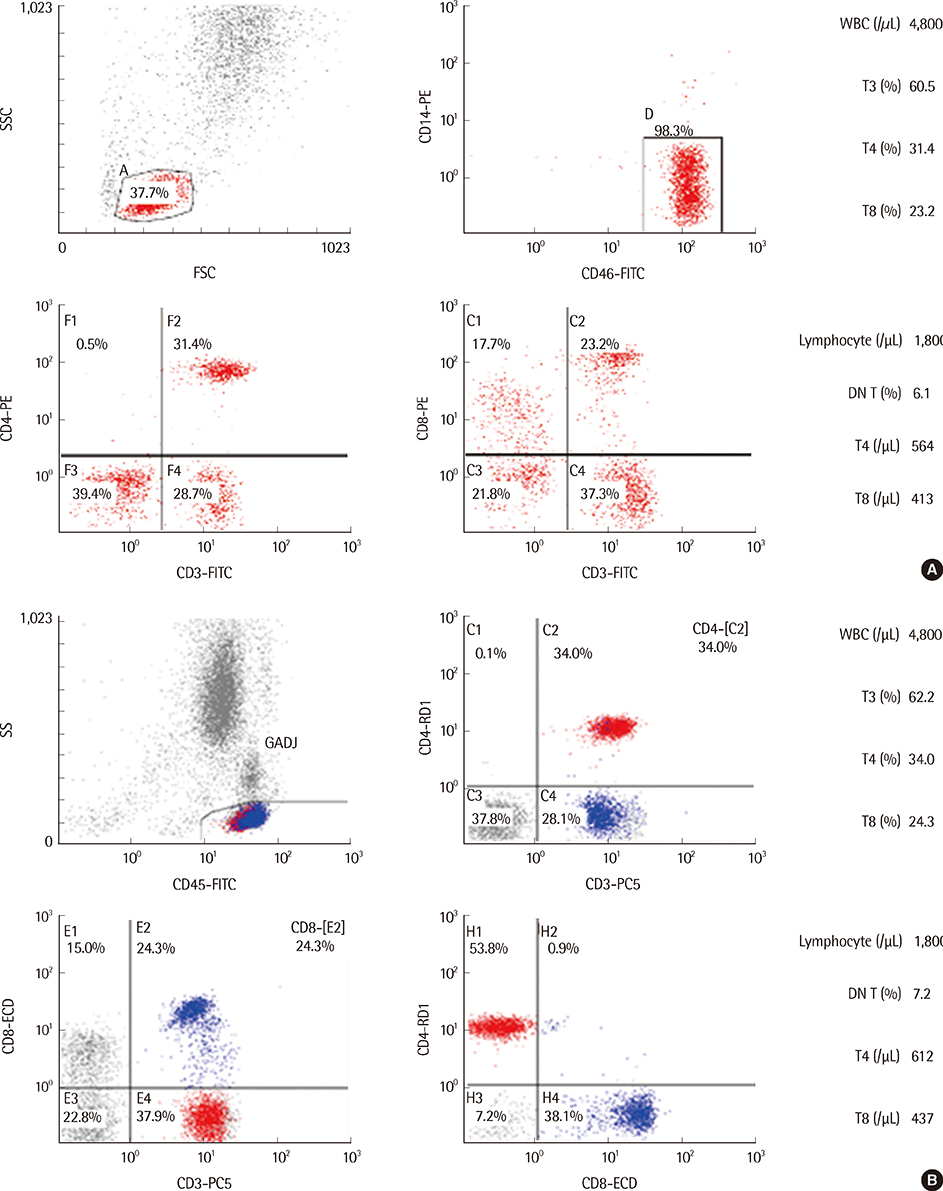
Blood CD4+ T-lymphocyte (T4) count is a major clinical marker for the diagnosis and management of AIDS, and flow cytometry is considered the gold standard for T4 enumeration. Our aim was to compare the 2-color and 4-color flow cytometric methods for T-cell subset analysis in HIV-infected patients.
METHODS
T-cell subsets such as T3, T4, T8, and CD3+CD4-CD8- double negative T cells (DN T) were analyzed from the whole blood of 40 HIV-infected patients by using both 2-color and 4-color methods on a Cytomics FC500 analyzer. Statistical analyses using simple linear regression, paired t-tests, and Bland-Altman plots were performed.
RESULTS
The measured T3 (%), T4 (%), T4 (/microL), T8 (%), T8 (/microL), and DN T (%) differed significantly between the 2 methods (P<0.05), whereas the T4/T8 ratio did not. T3 (%), T4 (%), T4 (/microL), T8 (%), T8 (/microL), and T4/T8 measured by the 2 methods showed good correlation, with correlation coefficients above 0.96, whereas DN T (%) did not. The mean differences in T4 (%) and T8 (%) were 0.39% (limit of agreement (LoA), -1.64~2.43) and 1.26% (LoA, -3.37~5.89), respectively.
CONCLUSIONS
Although there were statistically significant differences in the T cell subsets measured between the 2 methods, the differences were minor, and the 2 methods showed good correlation. As confirmed in this study, DN T (%) estimated by the 2-color method is lower than the actual value. We suggest that although the 2 methods can be used interchangeably, the 4-color method is recommended for the analysis of some specific subpopulations such as DN T (%).
Keyword
Figure
Reference
-
1. Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990; 322:166–172.
Article2. Fauci AS, Macher AM, Longo DL, Lane HC, Rook AH, Masur H, et al. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic consideration. Ann Intern Med. 1984; 100:92–106.
Article3. Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS society-USA panel. JAMA. 2008; 300:555–570.4. O'Brien WA, Hartigan PM, Martin D, Esinhart J, Hill A, Benoit S, et al. Veterans Affairs Cooperative Study Group on AIDS. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996; 334:426–431.5. World Health Organization. Laboratory guidelines for enumerating CD4 T lymphocytes in the context of HIV/AIDS. New Delhi WHO Regional Office for South-East Asia: WHO press;2007. p. 62–83.6. Bergeron M, Nicholson JK, Phaneuf S, Ding T, Soucy N, Badley AD, et al. Selection of lymphocyte gating protocol has impact on the level of reliability of T-cell subsets in aging specimens. Cytometry. 2002; 50:53–61.
Article7. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310.
Article8. UNAIDS and World Health Organization. Progress on global access to HIV antiretroviral therapy: a report on "3 by 5" and beyond. Geneva: WHO press;2006. p. 24–25.9. Korea Centers for Disease Control and Prevention. Annual report on the notified HIV/AIDS in Korea. Chungwon: Korea Centers for Disease Control and Prevention;2012. p. 14.10. D'Acquisto F, Crompton T. CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol. 2011; 82:333–340.11. Sundaravaradan V, Mir KD, Sodora DL. Double-negative T cells during HIV/SIV infections: potential pinch hitters in the T-cell lineup. Curr Opin HIV AIDS. 2012; 7:164–171.12. Fletcher MA, Baron GC, Ashman MR, Fischl MA, Klimas NG. Use of whole blood methods in assessment of immune parameters in immunodeficiency states. Diagn Clin Immunol. 1987; 5:69–81.13. Nicholson JK, Jones BM, Hubbard M. CD4 T-lymphocyte determinations on whole blood specimens using a single-tube three-color assay. Cytometry. 1993; 14:685–689.
Article14. Nicholson J, Kidd P, Mandy F, Livnat D, Kagan J. Three-color supplement to the NIAID DAIDS guideline for flow cytometric immunophenotyping. Cytometry. 1996; 26:227–230.
Article15. Kutok JL, Roma AO, Lemire SJ, Dorfman DM. Four-color flow cytometric immunophenotypic determination of peripheral blood CD4+ T lymphocyte counts: a comparison of validity and cost-effectiveness with a two-color method. Am J Clin Pathol. 1998; 110:465–470.
Article16. Mbopi-Kéou FX, Mion S, Sagnia B, Bélec L. Validation of a single-platform, volumetric, CD45-assisted PanLeucogating Auto40 flow cytometer to determine the absolute number and percentages of CD4 T cells in resource-constrained settings using Cameroonian patients' samples. Clin Vaccine Immunol. 2012; 19:609–615.
Article17. Clinical and Laboratory Standards Institute. EP5-A2. Enumeration of immunologically defined cell populations by flow cytometry; approved guideline. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2007.18. Luider J, Cyfra M, Johnson P, Auer I. Impact of the new Beckman Coulter Cytomics FC 500 5-color flow cytometer on a regional flow cytometry clinical laboratory service. Lab Hematol. 2004; 10:102–108.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitation of CD34 Positive Hematopoietic Stem Cells in Cord Blood by Flow Cytometric Analysis: Comparison of 3 Color Method (ProCOUNTTM) and 2 Color Method
- Minimal Change of Lymphocyte Subsets in 24 Hours-Stored Whole Blood Sample
- The Altered Pattern of CD28 Expression on T Cell Subsets in HIV-Infected Koreans
- The Diagnostic Value of the Color Doppler Ultrasonography in Chronic Prostatitis
- Comparison of Power Doppler and Color Doppler Ultrasonography in the Detection of Intratesticular Blood Flow of Normal Infants