Lab Med Online.
2012 Oct;2(4):209-214.
Evaluation of an Automated Urine Flow Cytometer for Screening of Bacterial Contamination in Platelet Concentrates
- Affiliations
-
- 1Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea. leewk@knu.ac.kr
Abstract
- BACKGROUND
Bacterial contamination of blood products, particularly of platelet concentrates (PCs), is a major risk factor for infections caused by blood transfusion. Various methods for the detection of bacterial contamination in PCs are available or are under investigation. We evaluated the usefulness of the Sysmex UF-1000i urine flow cytometer (Sysmex Medical Electronics Co, Japan) for screening of bacterial contamination in PCs.
METHODS
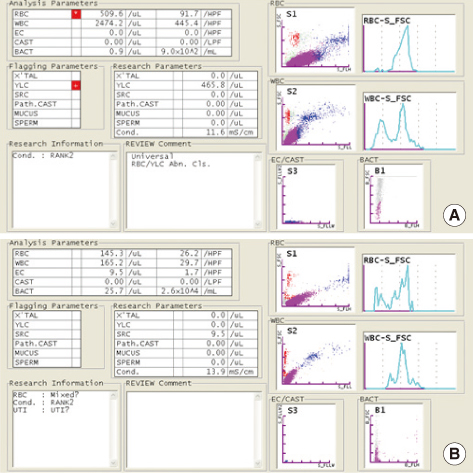
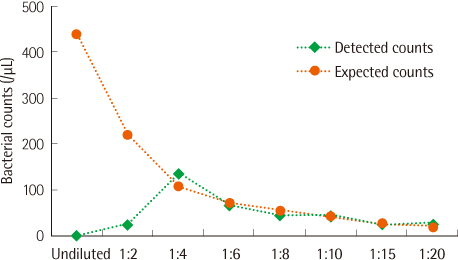
The PCs were inoculated with various concentrations of bacteria (Staphylococcus aureus and Escherichia coli) and were analyzed with the urine flow cytometer for bacterial counts. All the samples were diluted with normal saline (1:10) before flow cytometric analysis in order to prevent interference by the turbidity due to platelets.
RESULTS
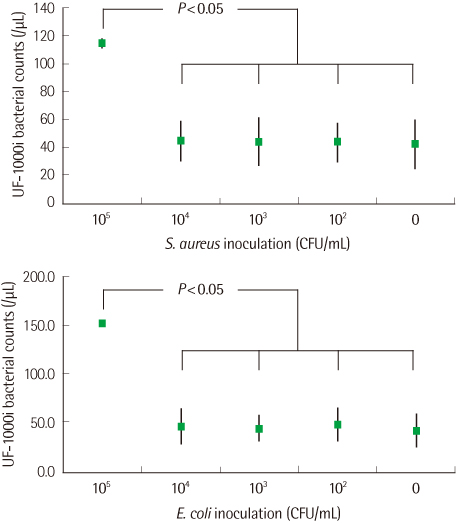
For PCs inoculated with a high number (colony forming unit, CFU) of bacteria (105 CFU/mL), the bacterial counts were significantly higher than those for uninoculated PCs analyzed by the urine flow cytometer. However, bacterial counts for PCs inoculated with bacteria of 104 CFU/mL or less and those for uninoculated PCs were not significantly different.
CONCLUSIONS
An automated urine flow cytometer evaluated in this study is easy to use, and the procedure is completed in less than 5 min. Moreover, the urine flow cytometer could detect approximately 105 CFU/mL of bacteria in PCs. Further validation studies are needed to assess the usefulness of this method for screening of bacterial contamination in PCs.
MeSH Terms
Figure
Reference
-
1. Kwon SY, Choi KY, Lim AH, Park KU, Cho NS. Comparison of methods for detecting bacterial contamination in platelet concentrates. Korean J Blood Transfus. 2011. 22:99–109.2. Hillyer CD, Josephson CD, Blajchman MA, Vostal JG, Epstein JS, Goodman JL. Bacterial contamination of blood components: risks, strategies, and regulation: joint ASH and AABB educational session in transfusion medicine. Hematology Am Soc Hematol Educ Program. 2003. 575–589.3. Blajchman MA, Goldman M, Baeza F. Improving the bacteriological safety of platelet transfusions. Transfus Med Rev. 2004. 18:11–24.
Article4. Park KU, Kwon SY, Kim SW, Lim YA. Long term prospects for the blood supply and demand. Korean J Blood Transfus. 2006. 17:1–10.5. Lee H, Park Y, Lim HS. Comparison of sensitivity of tests for detecting bacterial contamination in platelet concentrates. Korean J Blood Transfus. 2009. 20:32–39.6. Blood service statistics. 2011. Korean Red Cross;41.7. Kuehnert MJ, Roth VR, Haley NR, Gregory KR, Elder KV, Schreiber GB, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001. 41:1493–1499.
Article8. Pietersz RN, Engelfriet CP, Reesink HW, Wood EM, Winzar S, Keller AJ, et al. Detection of bacterial contamination of platelet concentrates. Vox Sang. 2007. 93:260–277.
Article9. Liu HW, Yuen KY, Cheng TS, Lee KB, Chua EK, Ho PL, et al. Reduction of platelet transfusion-associated sepsis by short-term bacterial culture. Vox Sang. 1999. 77:1–5.
Article10. Ramírez-Arcos S, Jenkins C, Dion J, Bernier F, Delage G, Goldman M. Canadian experience with detection of bacterial contamination in apheresis platelets. Transfusion. 2007. 47:421–429.
Article11. Blood service statistics. 2010. Korean Red Cross;78.12. Dreier J, Vollmer T, Kleesiek K. Novel flow cytometry-based screening for bacterial contamination of donor platelet preparations compared with other rapid screening methods. Clin Chem. 2009. 55:1492–1502.
Article13. Munksgaard L, Albjerg L, Lillevang ST, Gahrn-Hansen B, Georgsen J. Detection of bacterial contamination of platelet components: six years' experience with the BacT/ALERT system. Transfusion. 2004. 44:1166–1173.
Article14. Brecher ME, Hay SN, Rothenberg SJ. Evaluation of a new generation of plastic culture bottles with an automated microbial detection system for nine common contaminating organisms found in PLT components. Transfusion. 2004. 44:359–363.
Article15. Wagner SJ, Moroff G, Katz AJ, Friedman LI. Comparison of bacteria growth in single and pooled platelet concentrates after deliberate inoculation and storage. Transfusion. 1995. 35:298–302.
Article16. Goodnough LT, Shander A, Brecher ME. Transfusion medicine: looking to the future. Lancet. 2003. 361:161–169.
Article17. Jolkkonen S, Paattiniemi EL, Kärpänoja P, Sarkkinen H. Screening of urine samples by flow cytometry reduces the need for culture. J Clin Microbiol. 2010. 48:3117–3121.
Article18. Hu X, Zhang J, Zhang X. Evaluation of the Sysmex UF-1000i urine analyzer as a screening test to reduce the need for urine cultures for urinary tract infection. Labmedicine. 2010. 41:349–352.
Article19. Manoni F, Fornasiero L, Ercolin M, Tinello A, Ferrian M, Hoffer P, et al. Cutoff values for bacteria and leukocytes for urine flow cytometer Sysmex UF-1000i in urinary tract infections. Diagn Microbiol Infect Dis. 2009. 65:103–107.
Article20. Jacobs MR, Good CE, Lazarus HM, Yomtovian RA. Relationship between bacterial load, species virulence, and transfusion reaction with transfusion of bacterially contaminated platelets. Clin Infect Dis. 2008. 46:1214–1220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Sensitivity of Tests for Detecting Bacterial Contamination in Platelet Concentrates
- Comparison of Methods for Detecting Bacterial Contamination in Platelet Concentrates
- Selection of Unnecessary Urine Culture Specimens Using Sysmex UF-5000 Urine Flow Cytometer
- Evaluation of Sysmex UF-100 Urine Flow Cytometer in Predicting the Outcome of Urine Cultures
- Comparison of Analytical Performance between the Sysmex UF-100 flow cytometer and the Iris iQ200 Urine Microscopy System