Lab Anim Res.
2012 Mar;28(1):47-54. 10.5625/lar.2012.28.1.47.
Beneficial effects of melatonin on stroke-induced muscle atrophy in focal cerebral ischemic rats
- Affiliations
-
- 1Department of Rehabilitation Science in Interdisciplinary PhD Program, Graduate School of Inje University, Gimhae, Korea. yonghong@inje.ac.kr
- 2Department of Physical Therapy, College of Biomedical Science & Engineering, Inje University, Gimhae, Korea.
- 3National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang, Korea. changkt@kribb.re.kr
- 4Cardiovascular & Metabolic Disease Center, College of Biomedical Science & Engineering, Inje University, Gimhae, Korea.
- KMID: 2312092
- DOI: http://doi.org/10.5625/lar.2012.28.1.47
Abstract
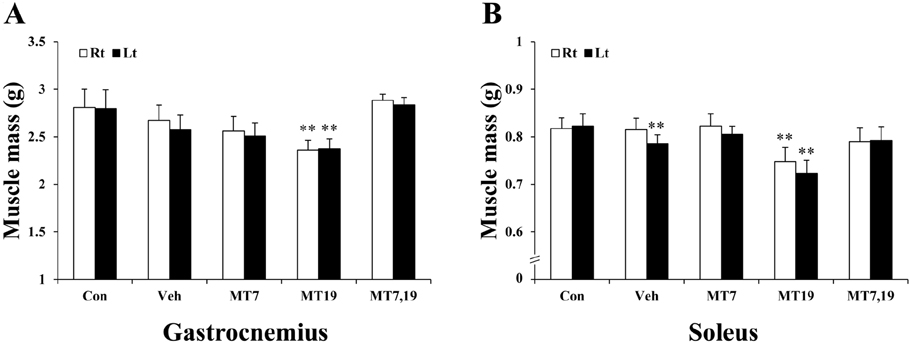
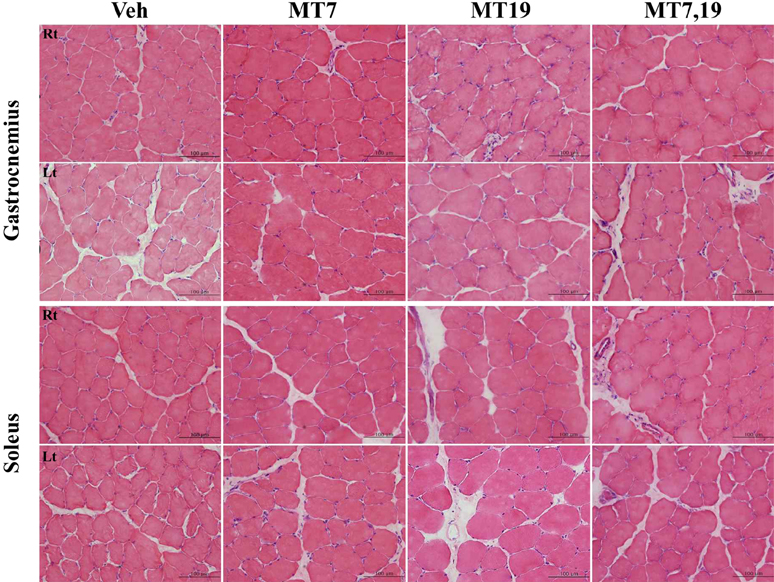
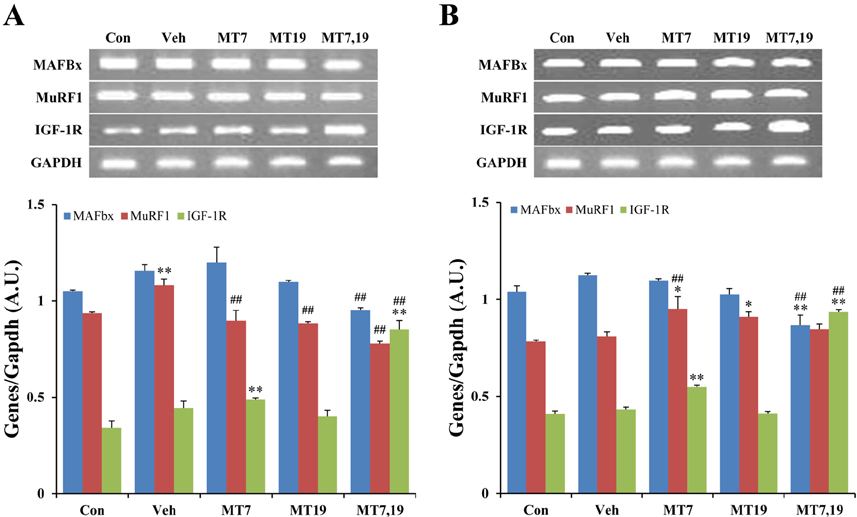
- Muscle atrophy is the result of two opposing conditions that can be found in pathological or diseased muscles: an imbalance in protein synthesis and degradation mechanisms. Thus, we investigated whether exogenous melatonin could regulate muscle components in stroke-induced muscle atrophy in rats. Comparing muscle phenotypes, we found that long-term melatonin administration could influence muscle mass. Muscle atrophy-related genes, including muscle atrophy F-box (MAFbx) and muscle ring finger 1 (MuRF1) were significantly down-regulated in melatonin-administered rats in the gastrocnemius. However, only MAFbx at the mRNA level was attenuated in the soleus of melatonin-administered rats. Insulin-like growth factor-1 receptor (IGF-1R) was significantly over-expressed in melatonin-administered rats in both the gastrocnemius and soleus muscles. Comparing myosin heavy chain (MHC) components, in the gastrocnemius, expression of both slow- and fast-type isoforms were significantly enhanced in melatonin-administered rats. These results suggest that long-term exogenous melatonin-administration may have a prophylactic effect on muscle atrophy through the MuRF1/MAFbx signaling pathway, as well as a potential therapeutic effect on muscle atrophy through the IGF-1-mediated hypertrophic signaling pathway in a stroke animal model.
Keyword
MeSH Terms
Figure
Reference
-
1. Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004. 287(4):C834–C843.2. Kasper CE, Talbot LA, Gaines JM. Skeletal muscle damage and recovery. AACN Clin Issues. 2002. 13(2):237–247.3. Tischler ME, Henriksen EJ, Munoz KA, Stump CS, Woodman CR, Kirby CR. Spaceflight on STS-48 and earth-based unweighting produce similar effects on skeletal muscle of young rats. J Appl Physiol. 1993. 74(5):2161–2165.4. Zeman RJ, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, Pan J, Bauman WA, Cardozo C. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch. 2009. 458(3):525–535.5. Drummond MJ, Glynn EL, Lujan HL, Dicarlo SE, Rasmussen BB. Gene and protein expression associated with protein synthesis and breakdown in paraplegic skeletal muscle. Muscle Nerve. 2008. 37(4):505–513.6. Nader GA, Hornberger TA, Esser KA. Translational control: implications for skeletal muscle hypertrophy. Clin Orthop Relat Res. 2002. (403 Suppl):S178–S187.7. Hafer-Macko CE, Ryan AS, Ivey FM, Macko RF. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev. 2008. 45(2):261–272.8. Boonyarom O, Inui K. Atrophy and hypertrophy of skeletal muscles: structural and functional aspects. Acta Physiol (Oxf). 2006. 188(2):77–89.9. McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004. 119(7):907–910.10. Hafer-Macko CE, Yu S, Ryan AS, Ivey FM, Macko RF. Elevated tumor necrosis factor-alpha in skeletal muscle after stroke. Stroke. 2005. 36(9):2021–2023.11. Scelsi R, Lotta S, Lommi G, Poggi P, Marchetti C. Hemiplegic atrophy: Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol. 1984. 62(4):324–331.12. Kernan WN, Inzucchi SE. Type 2 Diabetes Mellitus and Insulin Resistance: Stroke Prevention and Management. Curr Treat Options Neurol. 2004. 6(6):443–450.13. De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004. 30(2):209–215.14. Day CS, Buranapanitkit B, Riano FA, Tomaino MM, Somogyi G, Sotereanos DG, Kuroda R, Huard J. Insulin growth factor-1 decreases muscle atrophy following denervation. Microsurgery. 2002. 22(4):144–151.15. Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002. 83(12):1703–1707.16. Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc. 2004. 63(2):337–340.17. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998. 95(26):15603–15607.18. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005. 37(10):1974–1984.19. Altun A, Ugur-Altun B. Melatonin: therapeutic and clinical utilization. Int J Clin Pract. 2007. 61(5):835–845.20. Park S, Lee SK, Park K, Lee Y, Hong Y, Lee S, Jeon JC, Kim JH, Lee SR, Chang KT, Hong Y. Beneficial effects of endogenous and exogenous melatonin on neural reconstruction and functional recovery in an animal model of spinal cord injury. J Pineal Res. 2012. 52(1):107–119.21. Oner J, Oner H, Sahin Z, Demir R, Ustünel I. Melatonin is as effective as testosterone in the prevention of soleus muscle atrophy induced by castration in rats. Anat Rec (Hoboken). 2008. 291(4):448–455.22. Lee S, Shin J, Lee M, Lee S, Lee SR, Chang KT, Hong Y. Effects of melatonin on improvement of neurological function in focal cerebral ischemic rats. Reprod Dev Biol. 2011. 35(2):167–174.23. Lee S, Shin J, Lee M, Hong Y, Lee S, Lee Y, Lkhagvasuren T, Kim DW, Yang YA, Chang KT, Hong Y. The effects of melatonin and/or exercise on ischemic-reperfusion brain damage in focal cerebral ischemic rats. Neural Regen Res. 2012. In press.24. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011. 91(4):1447–1531.25. Lin XJ, Mei GP, Liu J, Li YL, Zuo D, Liu SJ, Zhao TB, Lin MT. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J Pineal Res. 2011. 50(4):436–444.26. Lee S, Lee HK, Shin J, Hong Y, Lee S, Lee S, Suzuki T, Kang T, Hong Y. Effects of controlled photoperiod on body development in growing juvenile rats. Reprod Dev Biol. 2010. 34(2):89–94.27. Gonciarz M, Gonciarz Z, Bielanski W, Mularczyk A, Konturek PC, Brzozowski T, Konturek SJ. The pilot study of 3-month course of melatonin treatment of patients with nonalcoholic steatohepatitis: effect on plasma levels of liver enzymes, lipids and melatonin. J Physiol Pharmacol. 2010. 61(6):705–710.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of DHEA on Hindlimb Muscles in a Focal Cerebral Ischemia Model Rat
- Animal Model of Cerebral Ischemia
- Induced Hypertension Using Phenylephrine in Patients with Acute Ischemic Stroke: A Case Report
- Effects of Isometric Contraction Training by Electrostimulation on Type I and II Hindlimb Muscles in Cerebral Ischemia Model Rats
- A ruptured aneurysmal subarachnoid hemorrhage and vasospasm initially manifesting as acute ischemic stroke: a case report