J Breast Cancer.
2016 Jun;19(2):163-168. 10.4048/jbc.2016.19.2.163.
Prognostically Distinctive Subgroup in Pathologic N3 Breast Cancer
- Affiliations
-
- 1Department of Surgery, Breast Cancer Center, Gachon University Gill Medical Center, Incheon, Korea. hgjh@gilhospital.com
- KMID: 2308967
- DOI: http://doi.org/10.4048/jbc.2016.19.2.163
Abstract
- PURPOSE
The aim of this retrospective study was to investigate whether there are prognostically different subgroups among patients with pathologic N3 (pN3) breast cancer.
METHODS
The records of 220 patients who underwent surgery for pN3 breast cancer from January 2006 to September 2012 were reviewed. All patients received adjuvant therapy according to standard protocols. The primary outcome was disease-free survival (DFS).
RESULTS
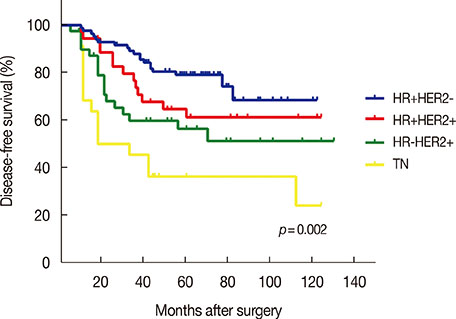
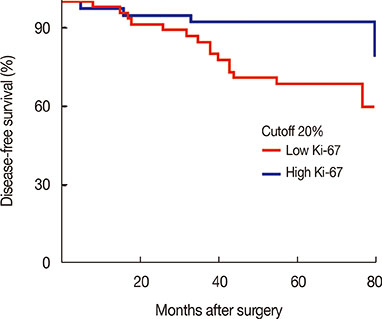
Patients were followed for a median time of 68.3 months after their primary surgery (range, 10-122 months), during which time 75 patients (34.1%) had developed disease recurrence and 48 patients (21.8%) had died. The DFS and overall survival were 67.8% and 86.1%, respectively, at 5 years. Multiple logistic regression analysis showed that young age (<35 years, p=0.009), high serum neutrophil/lymphocyte ratio (>3.0) (p=0.020), high nodal ratio (number of metastatic lymph nodes divided by number of removed nodes) (>0.65) (p=0.062), and molecular phenotype (p=0.012) were significantly associated with tumor recurrence. Tumor biological subtype was the most significant predictor of recurrence. The 5-year DFS rates in patients with hormone receptor (HR) positive and human epidermal growth factor receptor 2 (HER2) negative, HR+HER2+, HR-HER2+, and triple negative subtypes were 82%, 63%, 58%, and 37%, respectively.
CONCLUSION
Clinical outcomes of patients with extensive nodal metastasis were heterogeneous in terms of prognosis. Tumor biological subtype was the most important prognostic factor for pN3 disease. The prognosis of patients with HR+HER2- subtype in pN3 breast cancer was similar to that of patients with stage II breast cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Metastatic axillary node ratio predicts recurrence and poor long-term prognosis in patients with advanced stage IIIC (pN3) breast cancer
Min Hee Hur, SeungSang Ko
Ann Surg Treat Res. 2017;92(5):340-347. doi: 10.4174/astr.2017.92.5.340.
Reference
-
1. Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002; 20:3628–3636.
Article2. Kuru B, Camlibel M, Dinc S, Gulcelik MA, Alagol H. Prognostic significance of axillary node and infraclavicular lymph node status after mastectomy. Eur J Surg Oncol. 2003; 29:839–844.
Article3. Newman LA. Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009; 19:195–203.
Article4. Montero AJ, Rouzier R, Lluch A, Theriault RL, Buzdar AU, Delaloge S, et al. The natural history of breast carcinoma in patients with > or=10 metastatic axillary lymph nodes before and after the advent of adjuvant therapy: a multiinstitutional retrospective study. Cancer. 2005; 104:229–235.
Article5. Jones SE, Moon TE, Bonadonna G, Valagussa P, Rivkin S, Buzdar A, et al. Comparison of different trials of adjuvant chemotherapy in stage II breast cancer using a natural history data base. Am J Clin Oncol. 1987; 10:387–395.
Article6. Basaran G, Devrim C, Caglar HB, Gulluoglu B, Kaya H, Seber S, et al. Clinical outcome of breast cancer patients with N3a (≥10 positive lymph nodes) disease: has it changed over years? Med Oncol. 2011; 28:726–732.
Article7. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013; 88:218–230.
Article8. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009; 27:1062–1068.
Article9. Yonemori K, Tsuta K, Shimizu C, Hatanaka Y, Hashizume K, Ono M, et al. Immunohistochemical expression of PTEN and phosphorylated Akt are not correlated with clinical outcome in breast cancer patients treated with trastuzumab-containing neo-adjuvant chemotherapy. Med Oncol. 2009; 26:344–349.
Article10. Regan MM, Pagani O, Francis PA, Fleming GF, Walley BA, Kammler R, et al. Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat. 2015; 154:275–286.
Article11. Duraker N, Caynak ZC, Bati B. Is there any prognostically different subgroup among patients with stage IIIC (any TN3M0) breast carcinoma? Ann Surg Oncol. 2008; 15:430–437.
Article12. Diab SG, Hilsenbeck SG, de Moor C, Clark GM, Osborne CK, Ravdin PM, et al. Radiation therapy and survival in breast cancer patients with 10 or more positive axillary lymph nodes treated with mastectomy. J Clin Oncol. 1998; 16:1655–1660.
Article13. Duraker N, Bati B, Çaynak ZC, Demir D. Lymph node ratio may be supplementary to TNM nodal classification in node-positive breast carcinoma based on the results of 2,151 patients. World J Surg. 2013; 37:1241–1248.
Article14. Hong J, Mao Y, Chen X, Zhu L, He J, Chen W, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol. 2016; 37:4135–4142.
Article15. Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, et al. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2016; 37:361–368.
Article16. Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011; 13:R22.
Article17. Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008; 26:5569–5575.
Article18. Criscitiello C, Disalvatore D, De Laurentiis M, Gelao L, Fumagalli L, Locatelli M, et al. High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast. 2014; 23:69–75.
Article19. Kim KI, Lee KH, Kim TR, Chun YS, Lee TH, Park HK. Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2014; 17:40–46.
Article20. Kontzoglou K, Palla V, Karaolanis G, Karaiskos I, Alexiou I, Pateras I, et al. Correlation between Ki67 and breast cancer prognosis. Oncology. 2013; 84:219–225.
Article21. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.
Article22. Bagaria SP, Ray PS, Sim MS, Ye X, Shamonki JM, Cui X, et al. Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg. 2014; 149:125–129.
Article23. Jung HA, Park YH, Kim M, Kim S, Chang WJ, Choi MK, et al. Prognostic relevance of biological subtype overrides that of TNM staging in breast cancer: discordance between stage and biology. Tumour Biol. 2015; 36:1073–1079.
Article24. Veronesi U, Zurrida S, Viale G, Galimberti V, Arnone P, Nolè F. Rethinking TNM: a breast cancer classification to guide to treatment and facilitate research. Breast J. 2009; 15:291–295.
Article25. Yi M, Mittendorf EA, Cormier JN, Buchholz TA, Bilimoria K, Sahin AA, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011; 29:4654–4661.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Development of a Nomogram to Predict N2 or N3 Stage in T1-2 Invasive Breast Cancer Patients with No Palpable Lymphadenopathy
- The Prognosis of Breast Cancer Patients with 10 or more Positive Lymph Nodes
- Development of a Nomogram to Predict N2 or N3 Stage in T1–2 Invasive Breast Cancer Patients with No Palpable Lymphadenopathy
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?