J Clin Neurol.
2011 Sep;7(3):137-142. 10.3988/jcn.2011.7.3.137.
Efficacy and Safety of Switching from Oral Cholinesterase Inhibitors to the Rivastigmine Transdermal Patch in Patients with Probable Alzheimer's Disease
- Affiliations
-
- 1Department of Neurology, Myongji Hospital, Kwandong University of College of Medicine, Goyang, Korea.
- 2Department of Neurology, Sejong General Hospital, Bucheon, Korea.
- 3Department of Neurology, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 4Department of Neurology, Wonkwang University College of Medicine, Iksan, Korea.
- 5Department of Neurology, Ewha Womans University College of Medicine, Mokdong Hospital, Seoul, Korea.
- 6Department of Neurology, Bucheon St. Mary's Hospital, The Catholic University College of Medicine, Bucheon, Korea.
- 7Department of Neurology, Pusan National University Hospital and Medical Research Institute, Busan, Korea.
- 8Department of Neurology, Eulji University College of Medicine, Daejeon, Korea.
- 9Department of Neurology, Inha University College of Medicine, Incheon, Korea. seonghye@inha.ac.kr
- KMID: 2287628
- DOI: http://doi.org/10.3988/jcn.2011.7.3.137
Abstract
- BACKGROUND AND PURPOSE
The goal of this study was to estimate the efficacy and safety of the rivastigmine transdermal patch in patients with probable Alzheimer's disease (AD) who cannot tolerate or do not respond to oral cholinesterase inhibitors (ChEIs).
METHODS
A 24-week, prospective, open-label, single-arm, multicenter study was conducted from June 2009 to June 2010 in patients with probable AD. The enrolled patients had either a poor response or a decline in global function after treatment with oral ChEIs, or they were not able to tolerate treatment with oral ChEIs due to adverse events such as nausea or vomiting. A poor response was defined as a decrease of at least 2 points on the Korean version of the Mini-Mental State Examination (K-MMSE) within the previous 6 months (the decline in global function was determined by the investigator or caregiver). The efficacy of treatment was assessed using a follow-up Clinical Global Impression of Change (CGIC) assessment and K-MMSE conducted after 24 weeks, and safety was measured by the occurrence of adverse events and patient disposition.
RESULTS
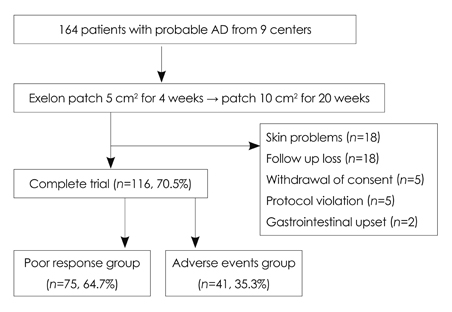
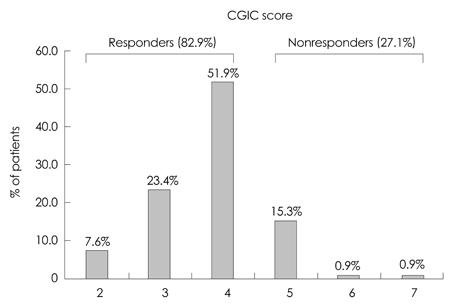
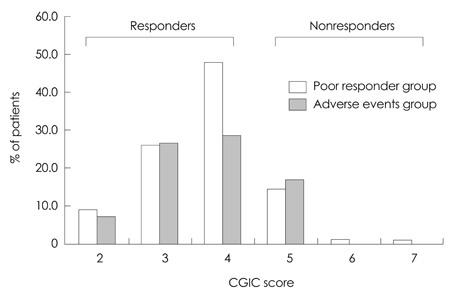
In total, 164 patients aged 74.7+/-7.52 years (mean+/-SD) and with 5.12+/-3.64 years of education were included. The study was completed by 70% of the patients (n=116), with 12.2% discontinuing due to adverse events. The most frequently reported adverse events (11%) were skin lesions, such as erythema or itching, followed by gastrointestinal problems (1.2%). Either an improvement or no decline in CGIC scores was reported for 82% of the patients.
CONCLUSIONS
The immediate switching of patients from an oral ChEI to the rivastigmine transdermal patch without a washout period was safe and well tolerated by the probable-AD patients in this study.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Effect of Rivastigmine Transdermal Patch on Sleep Apnea in Patients with Probable Alzheimer's Disease
Hyeyun Kim, Hyun Jeong Han
Dement Neurocogn Disord. 2016;15(4):153-158. doi: 10.12779/dnd.2016.15.4.153.The Effect of Rivastigmine Transdermal Patch on Sleep Apnea in Patients with Probable Alzheimer's Disease
Hyeyun Kim, Hyun Jeong Han
Dement Neurocogn Disord. 2016;15(4):153-158. doi: 10.12779/dnd.2016.15.4.153.
Reference
-
1. Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001. 56:1154–1166.
Article2. Ritchie CW, Ames D, Clayton T, Lai R. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psychiatry. 2004. 12:358–369.
Article3. Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ. 2005. 331:321–327.
Article4. Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer's disease. CNS Drugs. 2001. 15:375–390.
Article5. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002. 45–63.6. Small G, Dubois B. A review of compliance to treatment in Alzheimer's disease: potential benefits of a transdermal patch. Curr Med Res Opin. 2007. 23:2705–2713.
Article7. Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer's disease. Expert Opin Drug Deliv. 2008. 5:1377–1386.
Article8. Cummings J, Lefèvre G, Small G, Appel-Dingemanse S. Pharmacokinetic rationale for the rivastigmine patch. Neurology. 2007. 69:S10–S13.
Article9. Blesa R, Ballard C, Orgogozo JM, Lane R, Thomas SK. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer disease. Neurology. 2007. 69:S23–S28.
Article10. Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005. 105:145–158.11. Gauthier S, Emre M, Farlow MR, Bullock R, Grossberg GT, Potkin SG. Strategies for continued successful treatment of Alzheimer's disease: switching cholinesterase inhibitors. Curr Med Res Opin. 2003. 19:707–714.
Article12. Bullock R, Connolly C. Switching cholinesterase inhibitor therapy in Alzheimer's disease--donepezil to rivastigmine, is it worth it? Int J Geriatr Psychiatry. 2002. 17:288–289.
Article13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984. 34:939–944.
Article14. Kang Y, Na DL, Hahn S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997. 15:300–308.15. Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001. 56:1143–1153.
Article16. Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology. 1976. Bethesda, MD: National Institute of Mental Health.17. Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH, et al. The reliability and validity of the Korean instrumental activities of daily living (K-IADL). J Korean Neurol Assoc. 2002. 20:8–14.18. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001. 19:585–591.19. Auriacombe S, Pere JJ, Loria-Kanza Y, Vellas B. Efficacy and safety of rivastigmine in patients with Alzheimer's disease who failed to benefit from treatment with donepezil. Curr Med Res Opin. 2002. 18:129–138.
Article20. Sadowsky CH, Farlow MR, Atkinson L, Steadman J, Koumaras B, Chen M, et al. Switching from donepezil to rivastigmine is well tolerated: results of an open-label safety and tolerability study. Prim Care Companion J Clin Psychiatry. 2005. 7:43–48.21. Dantoine T, Auriacombe S, Sarazin M, Becker H, Pere JJ, Bourdeix I. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer's disease who failed to benefit from previous cholinesterase inhibitor treatment. Int J Clin Pract. 2006. 60:110–118.
Article22. Grossberg G, Sadowsky C, Fröstl H, Frölich L, Nagel J, Tekin S, et al. Safety and tolerability of the rivastigmine patch: results of a 28-week open-label extension. Alzheimer Dis Assoc Disord. 2009. 23:158–164.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Rivastigmine Transdermal Patch on Sleep Apnea in Patients with Probable Alzheimer's Disease
- Two Cases of Discontinuation Syndrome Following Cessation of Cholinesterase Inhibitors
- Recent Advances in Diagnosis and Treatment of Alzheimer's Disease
- Drug Therapy for Alzheimer's Disease
- Atypical Presentation of Acetylcholinesterase Inhibitor-Induced Diarrhea in Older Adults with Cognitive Decline: An Aspect not to be Underestimated