Korean J Physiol Pharmacol.
2014 Apr;18(2):135-141. 10.4196/kjpp.2014.18.2.135.
The Downregulation of Somatic A-Type K+ Channels Requires the Activation of Synaptic NMDA Receptors in Young Hippocampal Neurons of Rats
- Affiliations
-
- 1Department of Physiology, School of Medicine, Jeju National University, Jeju 690-756, Korea. jungsc@jejunu.ac.kr
- 2Institute of Medical Science, Jeju National University, Jeju 690-756, Korea.
- KMID: 2285502
- DOI: http://doi.org/10.4196/kjpp.2014.18.2.135
Abstract
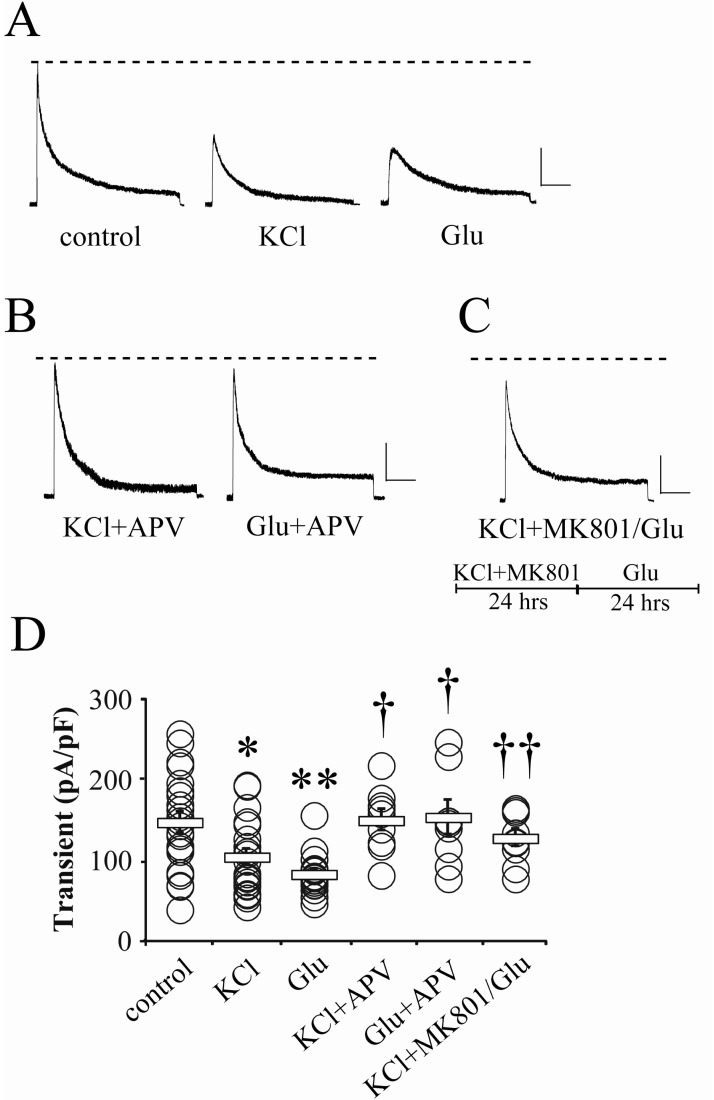
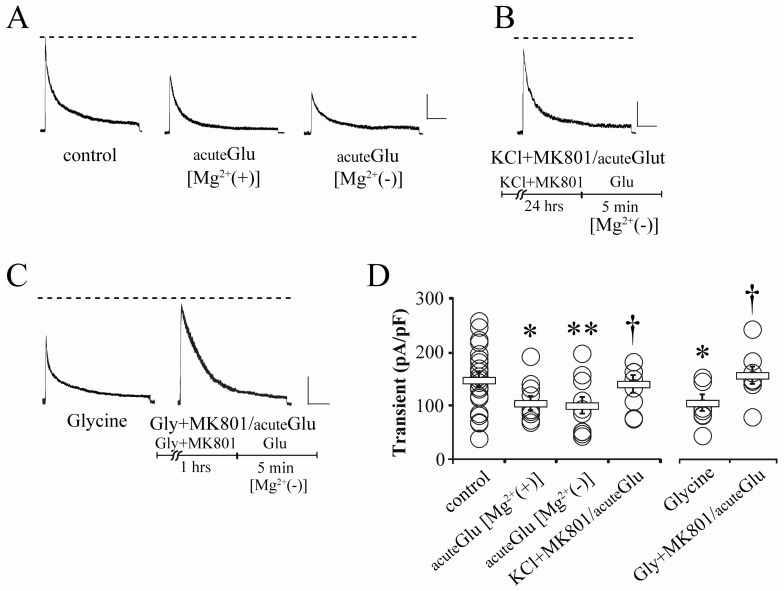
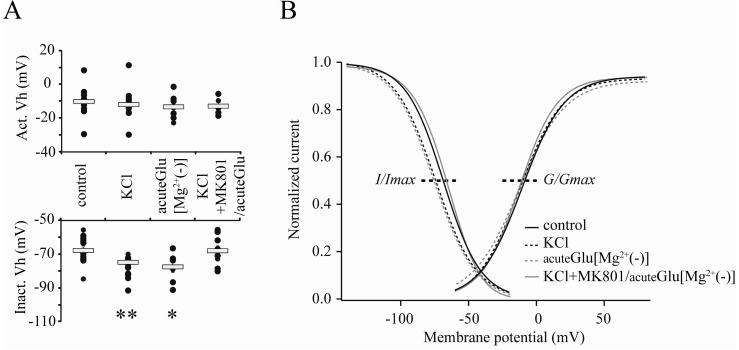
- The downregulation of A-type K+ channels (IA channels) accompanying enhanced somatic excitability can mediate epileptogenic conditions in mammalian central nervous system. As IA channels are dominantly targeted by dendritic and postsynaptic processings during synaptic plasticity, it is presumable that they may act as cellular linkers between synaptic responses and somatic processings under various excitable conditions. In the present study, we electrophysiologically tested if the downregulation of somatic IA channels was sensitive to synaptic activities in young hippocampal neurons. In primarily cultured hippocampal neurons (DIV 6~9), the peak of IA recorded by a whole-cell patch was significantly reduced by high KCl or exogenous glutamate treatment to enhance synaptic activities. However, the pretreatment of MK801 to block synaptic NMDA receptors abolished the glutamate-induced reduction of the IA peak, indicating the necessity of synaptic activation for the reduction of somatic IA. This was again confirmed by glycine treatment, showing a significant reduction of the somatic IA peak. Additionally, the gating property of IA channels was also sensitive to the activation of synaptic NMDA receptors, showing the hyperpolarizing shift in inactivation kinetics. These results suggest that synaptic LTP possibly potentiates somatic excitability via downregulating IA channels in expression and gating kinetics. The consequential changes of somatic excitability following the activity-dependent modulation of synaptic responses may be a series of processings for neuronal functions to determine outputs in memory mechanisms or pathogenic conditions.
Keyword
MeSH Terms
Figure
Reference
-
1. Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000; 3:109–111. PMID: 10649564.
Article2. Campanac E, Daoudal G, Ankri N, Debanne D. Downregulation of dendritic I(h) in CA1 pyramidal neurons after LTP. J Neurosci. 2008; 28:8635–8643. PMID: 18716222.
Article3. Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003; 4:885–900. PMID: 14595400.
Article4. Cash S, Yuste R. Input summation by cultured pyramidal neurons is linear and position-independent. J Neurosci. 1998; 18:10–15. PMID: 9412481.
Article5. Goldberg JH, Tamas G, Yuste R. Ca2+ imaging of mouse neocortical interneurone dendrites: IA-type K+ channels control action potential backpropagation. J Physiol. 2003; 551:49–65. PMID: 12844506.6. Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997; 387:869–875. PMID: 9202119.7. Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005; 569:41–57. PMID: 16141270.
Article8. Ramakers GM, Storm JF. A postsynaptic transient K+ current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 2002; 99:10144–10149. PMID: 12114547.9. Schoppa NE, Westbrook GL. Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nat Neurosci. 1999; 2:1106–1113. PMID: 10570488.
Article10. Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006; 26:12143–12151. PMID: 17122039.11. Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004; 7:126–135. PMID: 14730307.
Article12. Jung SC, Hoffman DA. Biphasic somatic A-type K+ channel downregulation mediates intrinsic plasticity in hippocampal CA1 pyramidal neurons. PLoS One. 2009; 4:e6549. PMID: 19662093.13. Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008; 60:657–671. PMID: 19038222.14. Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007; 54:933–947. PMID: 17582333.15. Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002; 99:8366–8371. PMID: 12048251.16. Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007; 56:582–592. PMID: 18031678.
Article17. Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010; 30:4590–4600. PMID: 20357110.
Article18. Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010; 30:2676–2685. PMID: 20164351.
Article19. Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006; 95:1727–1734. PMID: 16319212.
Article20. Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001; 29:243–254. PMID: 11182095.
Article21. Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci. 1998; 18:965–974. PMID: 9437018.
Article22. Kampa BM, Stuart GJ. Calcium spikes in basal dendrites of layer 5 pyramidal neurons during action potential bursts. J Neurosci. 2006; 26:7424–7432. PMID: 16837590.
Article23. Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008; 452:436–441. PMID: 18368112.
Article24. Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992; 9:271–284. PMID: 1497894.25. Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973; 32:331–356. PMID: 4727084.
Article26. Campanac E, Debanne D. Spike timing-dependent plasticity: a learning rule for dendritic integration in rat CA1 pyramidal neurons. J Physiol. 2008; 586:779–793. PMID: 18048448.
Article27. Lingamaneni R, Hemmings HC Jr. Effects of anticonvulsants on veratridine- and KCl-evoked glutamate release from rat cortical synaptosomes. Neurosci Lett. 1999; 276:127–130. PMID: 10624808.
Article28. Lei Z, Deng P, Li Y, Xu ZC. Downregulation of Kv4.2 channels mediated by NR2B-containing NMDA receptors in cultured hippocampal neurons. Neuroscience. 2010; 165:350–362. PMID: 19857555.
Article29. Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005; 47:101–114. PMID: 15996551.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Brain-derived Neurotrophic Factor on Excitatory Synaptic Transmission in Hippocampal Neurons
- The Role of NMDA Receptor in Learning and Memory
- Effect of Developmental Lead Exposure on the Expression of Hippocampal NMDA Receptor Subunit mRNA
- Chronic renal failure induces cell death in rat hippocampal CA1 via upregulation of alphaCaMKII/NR2A synaptic complex and phosphorylated GluR1-containing AMPA receptor cascades
- Synaptic Localization of NMDA Receptor and Shank Protein in Hippocampal Neuron in Vitro