Korean J Physiol Pharmacol.
2012 Dec;16(6):399-404. 10.4196/kjpp.2012.16.6.399.
Inhibitory Effects of ECQ on Indomethacin-Induced Gastric Damage in Rats
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- KMID: 2285448
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.399
Abstract
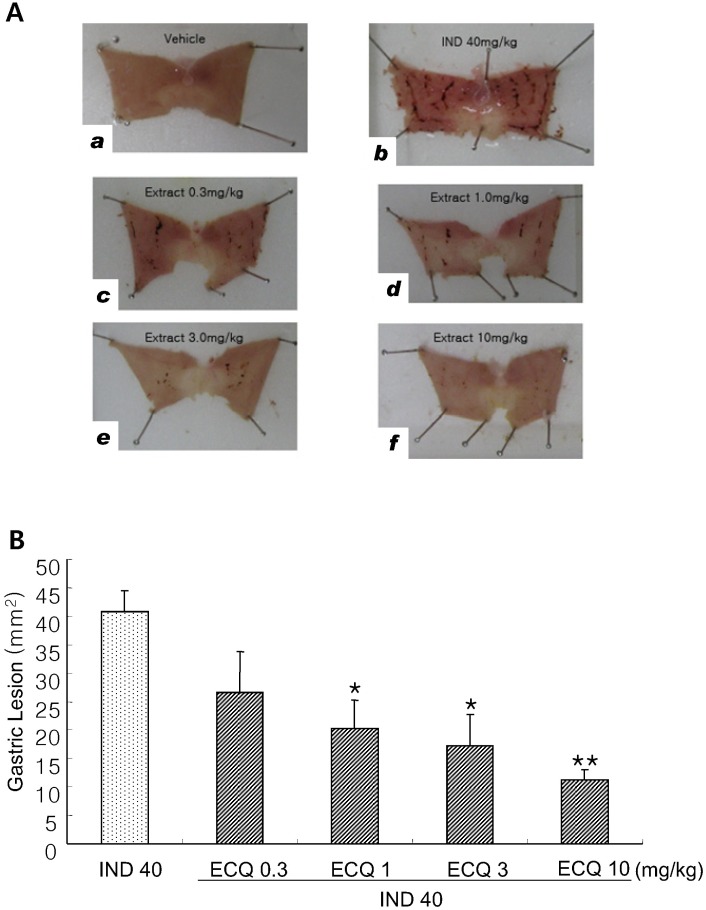
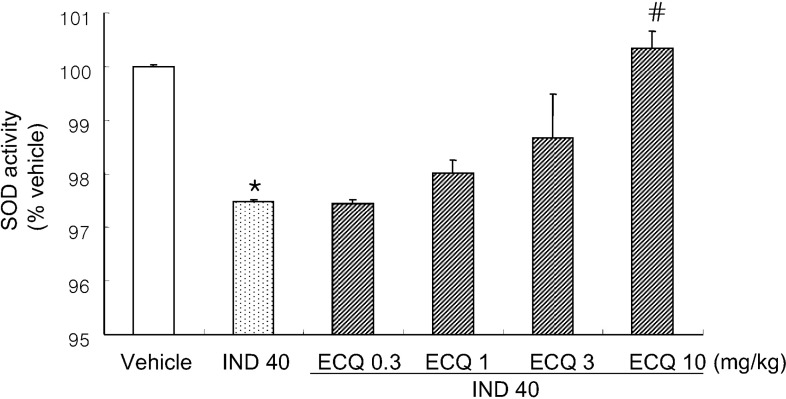
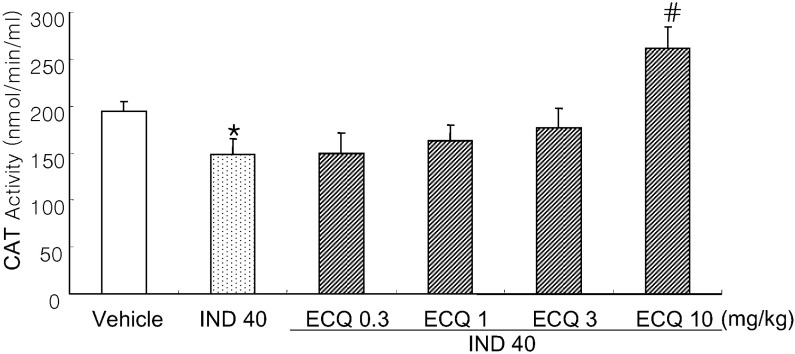
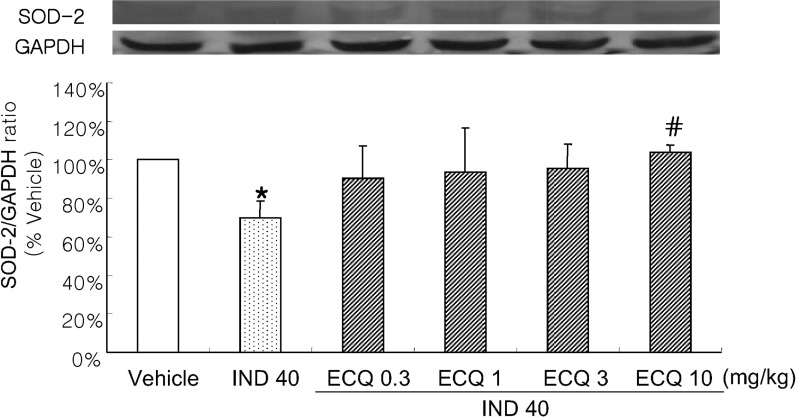
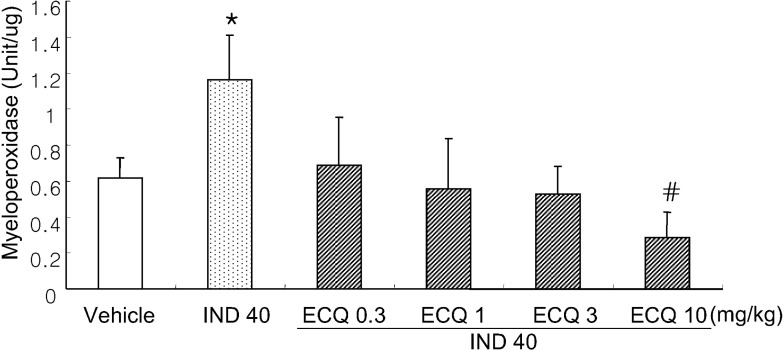
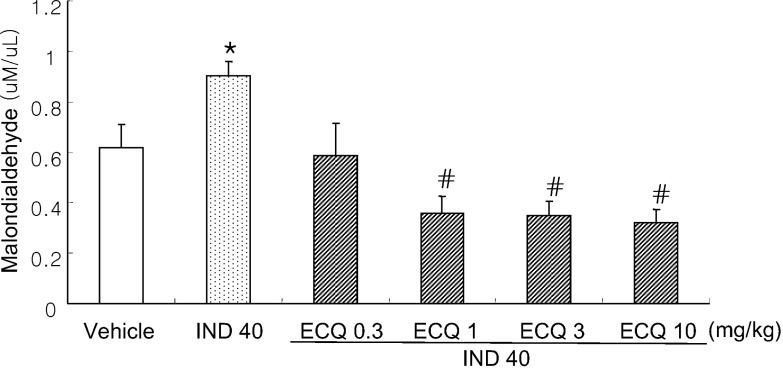
- We investigated inhibitory effects of extract containing quercetin-3-O-beta-D-glucuronopyranoside (ECQ) extracted from Rumex Aquaticus Herba on indomethacin-induced gastric damage in Rats. Gastritis was induced in male Sprague-Dawley rats (200~220 g) by oral administration of indomethacin at a dose of 40 mg/kg. One hour before administration of indomethacin, animals were orally pretreated with ECQ at doses of 0.3, 1, 3 or 10 mg/kg. Six hours after indomethacin administration, the rats were sacrificed and the stomach was excised and opened along the greater curvature, and the surface area of gastric lesion was measured using optical microscope. Superoxide dismutase (SOD), catalase (CAT), myeloperoxidase (MPO) activities and malondialdehyde (MDA) levels were measured by ELISA. Western blot analysis was performed to detect protein expression of SOD-2. Linear hemorrhagic mucosal lesions were observed in the stomach 6 hours after oral administration of indomethacin. Pretreatment with ECQ significantly reduced the severity of the lesions in a dose-dependent manner. It also inhibited the reductions in SOD and CAT activities and SOD expression by the indomethacin-induced gastric damage. In addition, the pretreatment with ECQ significantly suppressed the elevation of the MPO activity and the MDA levels induced by indomethacin. These results suggest that ECQ has the inhibitory effects via antioxidative action against indomethacin-induced gastritis in rats.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Oxytocin Ameliorates Remote Liver Injury Induced by Renal Ischemia-Reperfusion in Rats
Askın Tas Hekimoglu, Gulten Toprak, Hasan Akkoc, Osman Evliyaoglu, Selver Ozekinci, Ilker Kelle
Korean J Physiol Pharmacol. 2013;17(2):169-173. doi: 10.4196/kjpp.2013.17.2.169.
Reference
-
1. Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990; 259:G462–G467. PMID: 2169206.
Article2. Zhang X, Tajima K, Kageyama K, Kyoi T. Irsogladine maleate suppresses indomethacin-induced elevation of proinflammatory cytokines and gastric injury in rats. World J Gastroenterol. 2008; 14:4784–4790. PMID: 18720540.
Article3. Goenka MK, Majumder S, Sethy PK, Chakraborty M. Helicobacter pylori negative, non-steroidal anti-inflammatory drug-negative peptic ulcers in India. Indian J Gastroenterol. 2011; 30:33–37. PMID: 21424697.
Article4. Banerjee RK. Nonsteroidal anti-inflammatory drugs inhibit gastric peroxidase activity. Biochim Biophys Acta. 1990; 1034:275–280. PMID: 2163678.
Article5. Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med. 1997; 23:8–18. PMID: 9165292.
Article6. Abdallah DM, El-Abhar HS, Abdel-Aziz DH. TEMPOL, a membrane-permeable radical scavenger, attenuates gastric mucosal damage induced by ischemia/reperfusion: a key role for superoxide anion. Eur J Pharmacol. 2009; 603:93–97. PMID: 19087872.
Article7. Sugino N. The role of oxygen radical-mediated signaling pathways in endometrial function. Placenta. 2007; 28(Suppl A):S133–S136. PMID: 17291583.
Article8. Bandyopadhyay SK, Pakrashi SC, Pakrashi A. The role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin induced gastric ulcer. J Ethnopharmacol. 2000; 70:171–176. PMID: 10771207.
Article9. Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996; 87:1595–1599. PMID: 8608252.
Article10. Jainu M, Devi CS. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chem Biol Interact. 2006; 161:262–270. PMID: 16797507.
Article11. Kim Y, Kim WJ, Cha EJ. Quercetin-induced growth inhibition in human bladder cancer cells is associated with an increase in Ca-activated K Channels. Korean J Physiol Pharmacol. 2011; 15:279–283. PMID: 22128260.12. Lee BH, Shin TJ, Hwang SH, Choi SH, Kang J, Kim HJ, Park CW, Lee SH, Nah SY. Inhibitory effects of quercetin on muscle-type of nicotinic acetylcholine receptor-mediated ion currents expressed in xenopus oocytes. Korean J Physiol Pharmacol. 2011; 15:195–201. PMID: 21994477.
Article13. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000; 52:673–751. PMID: 11121513.14. Min YS, Lee SE, Hong ST, Kim HS, Choi BC, Sim SS, Whang WK, Sohn UD. The inhibitory effect of quercetin-3-O-beta-D-glucuronopyranoside on gastritis and reflux esophagitis in rats. Korean J Physiol Pharmacol. 2009; 13:295–300. PMID: 19885013.15. Kim JS, Song HJ, Ko SK, Whang WK, Sohn UD. Quercetin-3-O-beta-d-glucuronopyranoside (QGC)-induced HO-1 expression through ERK and PI3K activation in cultured feline esophageal epithelial cells. Fitoterapia. 2010; 81:85–92. PMID: 19686811.16. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52:302–310. PMID: 672633.17. Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990; 186:729–742. PMID: 2172726.18. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001; 74:418–425. PMID: 11566638.
Article19. Kostyuk VA, Potapovich AI, Speransky SD, Maslova GT. Protective effect of natural flavonoids on rat peritoneal macrophages injury caused by asbestos fibers. Free Radic Biol Med. 1996; 21:487–493. PMID: 8886799.
Article20. Martín MJ, La-Casa C, Alarcón-de-la-Lastra C, Cabeza J, Villegas I, Motilva V. Anti-oxidant mechanisms involved in gastroprotective effects of quercetin. Z Naturforsch C. 1998; 53:82–88. PMID: 9528125.
Article21. Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988; 37:837–841. PMID: 2830882.
Article22. Mascolo N, Pinto A, Capasso F. Flavonoids, leucocyte migration and eicosanoids. J Pharm Pharmacol. 1988; 40:293–295. PMID: 2900316.
Article23. Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure-activity relations. Biochem Pharmacol. 1984; 33:1525–1530. PMID: 6329230.24. Scambia G, Ranelletti FO, Benedetti Panici P, Piantelli M, Bonanno G, De Vincenzo R, Ferrandina G, Pierelli L, Capelli A, Mancuso S. Quercetin inhibits the growth of a multidrug-resistant estrogen-receptor-negative MCF-7 human breast-cancer cell line expressing type II estrogen-binding sites. Cancer Chemother Pharmacol. 1991; 28:255–258. PMID: 1879042.
Article25. Alarcón de la Lastra C, Martín MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin: a gross and histologic study. Pharmacology. 1994; 48:56–62. PMID: 8309988.
Article26. Suzuki Y, Ishihara M, Segami T, Ito M. Anti-ulcer effects of antioxidants, quercetin, alpha-tocopherol, nifedipine and tetracycline in rats. Jpn J Pharmacol. 1998; 78:435–441. PMID: 9920200.27. Yan XM, Joo MJ, Lim JC, Whang WK, Sim SS, Im C, Kim HR, Lee SY, Kim IK, Sohn UD. The effect of quercetin-3-O-β-D-glucuronopyranoside on indomethacin-induced gastric damage in rats via induction of mucus secretion and down-regulation of ICAM-1 expression. Arch Pharm Res. 2011; 34:1527–1534. PMID: 21975815.
Article28. Cho JH, Park SY, Lee HS, Whang WK, Sohn UD. The protective effect of quercetin-3-O-β-D-glucuronopyranoside on ethanol-induced damage in cultured feline esophageal epithelial cells. Korean J Physiol Pharmacol. 2011; 15:319–326. PMID: 22359468.
Article29. Kvietys PR, Twohig B, Danzell J, Specian RD. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 1990; 98:909–920. PMID: 2311875.30. Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990; 8:583–599. PMID: 2193855.
Article31. El-Missiry MA, El-Sayed IH, Othman AI. Protection by metal complexes with SOD-mimetic activity against oxidative gastric injury induced by indomethacin and ethanol in rats. Ann Clin Biochem. 2001; 38:694–700. PMID: 11732653.32. Motilva V, Martín MJ, Luque MI, Alarcón de la Lastra C. Role of polymorphonuclear leukocytes and oxygen-derived free radicals in chronic gastric lesion induced by acetic acid in rat. Gen Pharmacol. 1996; 27:545–550. PMID: 8723542.
Article33. Mart'ianov AA, Emel'ianova TG, Obukhova MF, Volkova NV, Riabinina MA, Vakulina OP, Sakharov IIu, Ashmarin IP. Physiologic effects of active immunization with triiodothyronine in rats. Biull Eksp Biol Med. 1998; 126:497–501. PMID: 9883352.34. Zimmerman BJ, Granger DN. Oxygen free radicals and the gastrointestinal tract: role in ischemia-reperfusion injury. Hepatogastroenterology. 1994; 41:337–342. PMID: 7959568.35. Naito Y, Yoshikawa T, Yoshida N, Kondo M. Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig Dis Sci. 1998; 43(9 Suppl):30S–34S. PMID: 9753223.36. Takeuchi K, Ueshima K, Hironaka Y, Fujioka Y, Matsumoto J, Okabe S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion. 1991; 49:175–184. PMID: 1769433.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Neutrophil on Nonsteroidal Anti-Inflammatory Drugs Induced Acute Gastric Mucosal Injury
- Protective Effect of ECQ on Rat Reflux Esophagitis Model
- The Inhibitory Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Gastritis and Reflux Esophagitis in Rats
- Effect of Meloxicam, Selective COX-2 Inhibitor, on Gastric Mucosal Damage and Serum TNF-alpha
- The Effects of Indomethacin of the Rat Gastric Mucosa