Korean J Physiol Pharmacol.
2012 Dec;16(6):455-462. 10.4196/kjpp.2012.16.6.455.
Protective Effect of ECQ on Rat Reflux Esophagitis Model
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- KMID: 2285455
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.455
Abstract
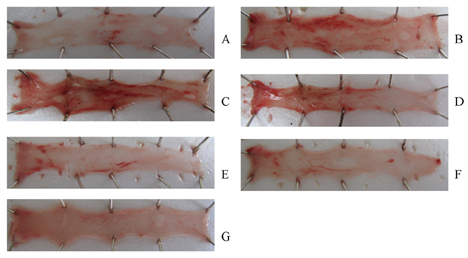
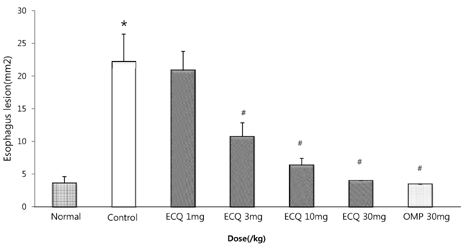
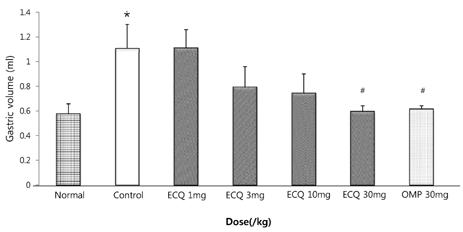
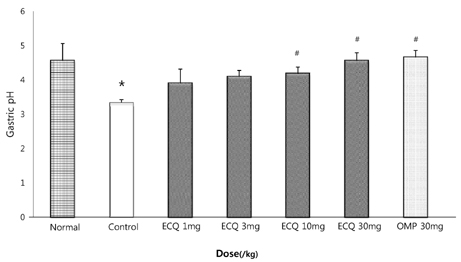
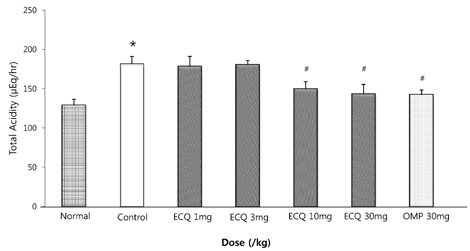
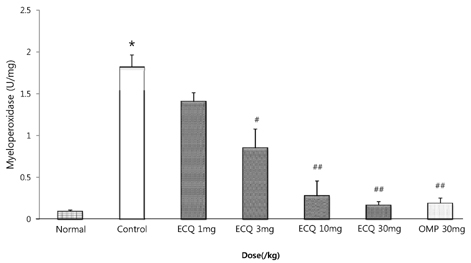
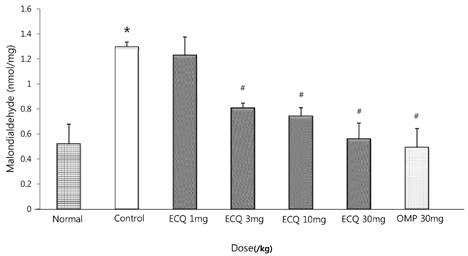
- This study was designed to determine the protective effect of Rumex Aquaticus Herba extracts containing quercetin-3-beta-D-glucuronopyranoside (ECQ) on experimental reflux esophagitis. Reflux esophagitis was induced by surgical procedure. The rats were divided into seven groups, namely normal group, control group, ECQ (1, 3, 10, 30 mg/kg) group and omeprazole (30 mg/kg) group. ECQ and omeprazole groups received intraduodenal administration. The Rats were starved for 24 hours before the experiments, but were freely allowed to drink water. ECQ group attenuated the gross esophagitis significantly compared to that treated with omeprazole in a dose-dependent manner. ECQ decreased the volume of gastric juice and increased the gastric pH, which are similar to those of omeprazole group. In addition, ECQ inhibited the acid output effectively in reflux esophagitis. Significantly increased amounts of malondialdehyde (MDA), myeloperoxidase (MPO) activity and the mucosal depletion of reduced glutathione (GSH) were observed in the reflux esophagitis. ECQ administration attenuated the decrement of the GSH levels and affected the MDA levels and MPO activity. These results suggest that the ECQ has a protective effect which may be attributed to its multiple effects including anti-secretory, anti-oxidative and anti-inflammatory actions on reflux esophagitis in rats.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Oxytocin Ameliorates Remote Liver Injury Induced by Renal Ischemia-Reperfusion in Rats
Askın Tas Hekimoglu, Gulten Toprak, Hasan Akkoc, Osman Evliyaoglu, Selver Ozekinci, Ilker Kelle
Korean J Physiol Pharmacol. 2013;17(2):169-173. doi: 10.4196/kjpp.2013.17.2.169.Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats
Minjae Joo, Han Sang Kim, Tae Hoon Kwon, Alisha Palikhe, Tin Sandar Zaw, Ji Hoon Jeong, Uy Dong Sohn
Korean J Physiol Pharmacol. 2015;19(1):43-50. doi: 10.4196/kjpp.2015.19.1.43.
Reference
-
1. Kim Y, Kim WJ, Cha EJ. Quercetin-induced growth inhibition in human bladder cancer cells is associated with an increase in ca-activated K channels. Korean J Physiol Pharmacol. 2011. 15:279–283.2. Nishida S, Satoh H. Possible involvement of Ca activated K channels, SK channel, in the quercetin-induced vasodilatation. Korean J Physiol Pharmacol. 2009. 13:361–365.3. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000. 52:673–751.4. Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004. 36:838–849.5. Haenen GR, Paquay JB, Korthouwer RE, Bast A. Peroxynitrite scavenging by flavonoids. Biochem Biophys Res Commun. 1997. 236:591–593.6. Heijnen CG, Haenen GR, van Acker FA, van der Vijgh WJ, Bast A. Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol In Vitro. 2001. 15:3–6.7. Diplock AT. Defense against reactive oxygen species. Free Radic Res. 1998. 29:463–467.8. Min YS, Lee SE, Hong ST, Kim HS, Choi BC, Sim SS, Whang WK, Sohn UD. The inhibitory effect of quercetin-3-O-beta-D-lucuronopyranoside on gastritis and reflux esophagitis in rats. Korean J Physiol Pharmacol. 2009. 13:295–300.9. Nakamura K, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 1982. 32:445–456.10. Bell NJ, Hunt RH. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut. 1992. 33:118–124.11. Wetscher GJ, Hinder RA, Klingler P, Gadenstätter M, Perdikis G, Hinder PR. Reflux esophagitis in humans is a free radical event. Dis Esophagus. 1997. 10:29–32.12. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001. 49:364–371.13. Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997. 40:175–181.14. Stein HJ, Hinder RA, Oosthuizen MM. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: protective role of the antioxidant glutathione. Surgery. 1990. 108:467–473.15. Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987. 32:1395–1401.16. Haegele AD, Briggs SP, Thompson HJ. Antioxidant status and dietary lipid unsaturation modulate oxidative DNA damage. Free Radic Biol Med. 1994. 16:111–115.17. Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K, Hinder RA. Free radical production in nicotine treated pancreatic tissue. Free Radic Biol Med. 1995. 18:877–882.18. Ljubicić N, Habijanić E, Antić Z, Doko M, Kovacević I, Zovak M. Medical treatment of gastroesophageal reflux disease. Acta Med Croatica. 1998. 52:133–138.19. Okabe S, Takinami Y, Iwata K, Yanagawa T. Mucosal protective effect of leminoprazole on reflux esophagitis induced in rats. Jpn J Pharmacol. 1995. 69:317–323.20. Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990. 186:729–742.21. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963. 61:882–888.22. Biancani P, Barwick K, Selling J, McCallum R. Effects of acute experimental esophagitis on mechanical properties of the lower esophageal sphincter. Gastroenterology. 1984. 87:8–16.23. Lo Piparo E, Scheib H, Frei N, Williamson G, Grigorov M, Chou CJ. Flavonoids for controlling starch digestion: structural requirements for inhibiting human alpha-amylase. J Med Chem. 2008. 51:3555–3561.24. Martín MJ, La-Casa C, Alarcón-de-la-Lastra C, Cabeza J, Villegas I, Motilva V. Anti-oxidant mechanisms involved in gastroprotective effects of quercetin. Z Naturforsch C. 1998. 53:82–88.25. Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988. 37:837–841.26. Kostyuk VA, Potapovich AI, Speransky SD, Maslova GT. Protective effect of natural flavonoids on rat peritoneal macrophages injury caused by asbestos fibers. Free Radic Biol Med. 1996. 21:487–493.27. Alarcón de la Lastra C, Martín MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin: a gross and histologic study. Pharmacology. 1994. 48:56–62.28. Mascolo N, Pinto A, Capasso F. Flavonoids, leucocyte migration and eicosanoids. J Pharm Pharmacol. 1988. 40:293–295.29. Landolfi R, Mower RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure-activity relations. Biochem Pharmacol. 1984. 33:1525–1530.30. Scambia G, Ranelletti FO, Benedetti Panici P, Piantelli M, Bonanno G, De Vincenzo R, Ferrandina G, Pierelli L, Capelli A, Mancuso S. Quercetin inhibits the growth of a multidrug-resistant estrogen-receptor-negative MCF-7 human breast-cancer cell line expressing type II estrogen-binding sites. Cancer Chemother Pharmacol. 1991. 28:255–258.31. Yang J, Liu RH. Synergistic effect of apple extracts and quercetin 3-beta-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem. 2009. 57:8581–8586.32. Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, Marino M. Quercetin-induced apoptotic cascade in cancer cells: antioxidant versus estrogen receptor alpha-dependent mechanisms. Mol Nutr Food Res. 2009. 53:699–708.33. Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N, Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anticancer Agents Med Chem. 2009. 9:138–161.34. Kim JS, Song HJ, Ko SK, Whang WK, Sohn UD. Quercetin-3-O-beta-d-glucuronopyranoside (QGC)-nduced HO-1 expression through ERK and PI3K activation in cultured feline esophageal epithelial cells. Fitoterapia. 2010. 81:85–92.35. Velázquez C, Calzada F, Esquivel B, Barbosa E, Calzada S. Antisecretory activity from the flowers of Chiranthodendron pentadactylon and its flavonoids on intestinal fluid accumulation induced by Vibrio cholerae toxin in rats. J Ethnopharmacol. 2009. 126:455–458.36. Ito M, Suzuki Y, Ishihara M, Suzuki Y. Anti-ulcer effects of antioxidants: effect of probucol. Eur J Pharmacol. 1998. 354:189–196.37. Conchillo JM, Schwartz MP, Selimah M, Samsom M, Sifrim D, Smout AJ. Acid and non-acid reflux patterns in patients with erosive esophagitis and non-erosive reflux disease (NERD): a study using intraluminal impedance monitoring. Dig Dis Sci. 2008. 53:1506–1512.38. da Rocha JR, Cecconello I, Ribeiro U Jr, Baba ER, Safatle-Ribeiro AV, Iriya K, Sallum RA, Sakai P, Szachnowicz S. Preoperative gastric acid secretion and the risk to develop Barrett's esophagus after esophagectomy for chagasic achalasia. J Gastrointest Surg. 2009. 13:1893–1898.39. Yasuda H, Yamada M, Endo Y, Inoue K, Yoshiba M. Acute necrotizing esophagitis: role of nonsteroidal anti-inflammatory drugs. J Gastroenterol. 2006. 41:193–197.40. Gerritsen ME, Carley WW, Ranges GE, Shen CP, Phan SA, Ligon GF, Perry CA. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995. 147:278–292.41. Panés J, Gerritsen ME, Anderson DC, Miyasaka M, Granger DN. Apigenin inhibits tumor necrosis factor-induced intercellular adhesion molecule-1 upregulation in vivo. Microcirculation. 1996. 3:279–286.42. Wetscher GJ, Hinder RA, Bagchi D, Hinder PR, Bagchi M, Perdikis G, McGinn T. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1995. 170:552–556.43. Anderson LA, Cantwell MM, Watson RG, Johnston BT, Murphy SJ, Ferguson HR, McGuigan J, Comber H, Reynolds JV, Murray LJ. The association between alcohol and reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma. Gastroenterology. 2009. 136:799–805.44. Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004. 62:1186–1197.45. Kubo A, Levin TR, Block G, Rumore GJ, Quesenberry CP Jr, Buffler P, Corley DA. Dietary antioxidants, fruits, and vegetables and the risk of Barrett's esophagus. Am J Gastroenterol. 2008. 103:1614–1623.46. Shirin H, Pinto JT, Liu LU, Merzianu M, Sordillo EM, Moss SF. Helicobacter pylori decreases gastric mucosal glutathione. Cancer Lett. 2001. 164:127–133.47. Rathinam ML, Watts LT, Stark AA, Mahimainathan L, Stewart J, Schenker S, Henderson GI. Astrocyte control of fetal cortical neuron glutathione homeostasis: up-regulation by ethanol. J Neurochem. 2006. 96:1289–1300.48. Lee JM, Im WJ, Nam YJ, Oh KH, Lim JC, Whang WK, Sohn UD. Acute toxicity and general pharmacological action of QGC EXT. Korean J Physiol Pharmacol. 2012. 16:49–57.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effect of quercetin and desferrioxamine in rat reflux esophagitis
- Relationship between Reflux Esophagitis and Helicobacter pylori Infection
- Reflux Esophagitis after Cure of Helicobacter pylori Infection: Report of two cases
- Minimal Change Esophagitis
- Gastroesophageal Reflux in Peptic Ulcer Patients