Korean J Physiol Pharmacol.
2011 Dec;15(6):415-422. 10.4196/kjpp.2011.15.6.415.
Spontaneous Oscillatory Rhythm in Retinal Activities of Two Retinal Degeneration (rd1 and rd10) Mice
- Affiliations
-
- 1Department of Physiology, Chungbuk National University School of Medicine, Korea. ysgoo@chungbuk.ac.kr
- 2Department of Physics, Chungbuk National University, Cheongju 361-763, Korea.
- 3Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju 220-710, Korea.
- KMID: 2285429
- DOI: http://doi.org/10.4196/kjpp.2011.15.6.415
Abstract
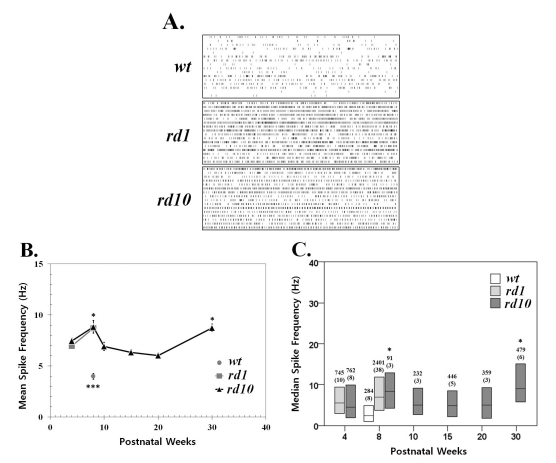
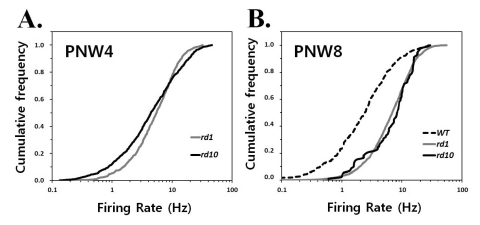
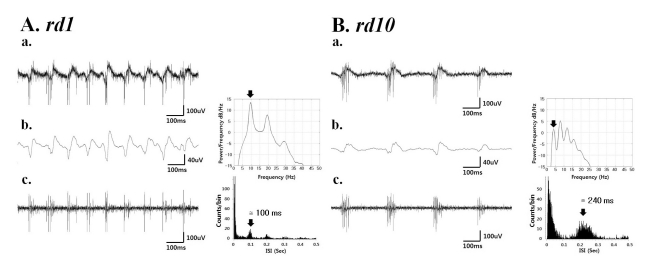
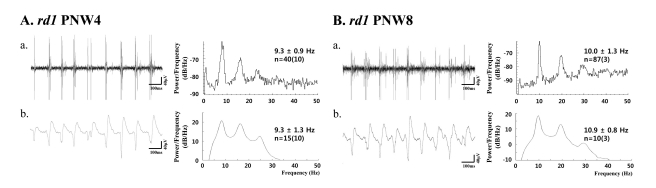
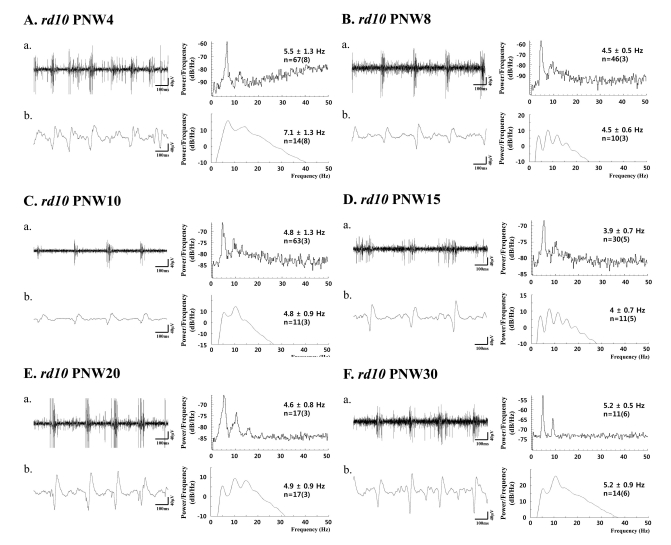
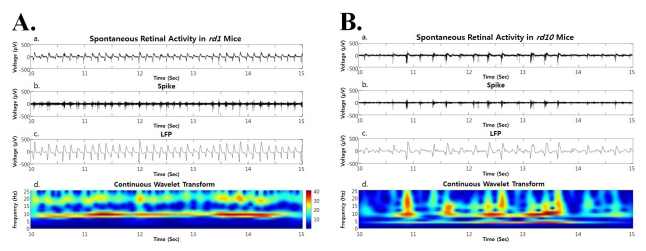
- Previously, we reported that besides retinal ganglion cell (RGC) spike, there is ~ 10 Hz oscillatory rhythmic activity in local field potential (LFP) in retinal degeneration model, rd1 mice. The more recently identified rd10 mice have a later onset and slower rate of photoreceptor degeneration than the rd1 mice, providing more therapeutic potential. In this study, before adapting rd10 mice as a new animal model for our electrical stimulation study, we investigated electrical characteristics of rd10 mice. From the raw waveform of recording using 8x8 microelectrode array (MEA) from in vitro-whole mount retina, RGC spikes and LFP were isolated by using different filter setting. Fourier transform was performed for detection of frequency of bursting RGC spikes and oscillatory field potential (OFP). In rd1 mice, ~10 Hz rhythmic burst of spontaneous RGC spikes is always phase-locked with the OFP and this phase-locking property is preserved regardless of postnatal ages. However, in rd10 mice, there is a strong phase-locking tendency between the spectral peak of bursting RGC spikes (~5 Hz) and the first peak of OFP (~5 Hz) across different age groups. But this phase-locking property is not robust as in rd1 retina, but maintains for a few seconds. Since rd1 and rd10 retina show phase-locking property at different frequency (~10 Hz vs. ~5 Hz), we expect different response patterns to electrical stimulus between rd1 and rd10 retina. Therefore, to extract optimal stimulation parameters in rd10 retina, first we might define selection criteria for responding rd10 ganglion cells to electrical stimulus.
Keyword
MeSH Terms
Figure
Reference
-
1. Chader GJ, Weiland J, Humayun MS. Artificial vision: needs, functioning, and testing of a retinal electronic prosthesis. Prog Brain Res. 2009; 175:317–332. PMID: 19660665.
Article2. Rizzo JF 3rd. Update on retinal prosthetic research: the Boston Retinal Implant Project. J Neuroophthalmol. 2011; 31:160–168. PMID: 21593628.
Article3. Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S, Koch J, Kusnyerik A, Peters T, Stingl K, Sachs H, Stett A, Szurman P, Wilhelm B, Wilke R. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011; 278:1489–1497. PMID: 21047851.
Article4. Farber DB, Flannery JG, Bowes-Rickman C. The rd mouse story: seventy years of research on an animal model of inherited retinal degeneration. Prog Retinal Eye Res. 1994; 13:31–64.
Article5. LaVail MM, Matthes MT, Yasumura D, Steinberg RH. Variability in rate of cone degeneration in the retinal degeneration (rd/rd) mouse. Exp Eye Res. 1997; 65:45–50. PMID: 9237863.
Article6. Pierce EA. Pathways to photoreceptor cell death in inherited retinal degenerations. Bioessays. 2001; 23:605–618. PMID: 11462214.
Article7. Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007; 47:624–633. PMID: 17267005.8. Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrowderived lineage-negative hematopoietic stem cells. J Clin Invest. 2004; 114:765–774. PMID: 15372100.
Article9. Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther. 2004; 10:855–861. PMID: 15509503.
Article10. Picard E, Jonet L, Sergeant C, Vesvres MH, Behar-Cohen F, Courtois Y, Jeanny JC. Overexpressed or intraperitoneally injected human transferrin prevents photoreceptor degeneration in rd10 mice. Mol Vis. 2010; 16:2612–2625. PMID: 21179240.11. Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S, Everhart D, Dinculescu A, Liu L, Umino Y, Lei B, Chang B, Barlow R, Strettoi E, Hauswirth WW. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011; 19:234–242. PMID: 21139570.
Article12. Ye JH, Goo YS. The slow wave component of retinal activity in rd/rd mice recorded with a multi-electrode array. Physiol Meas. 2007; 28:1079–1088. PMID: 17827655.13. Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol. 2008; 99:1408–1421. PMID: 18216234.
Article14. Margolis DJ, Newkirk G, Euler T, Detwiler PB. Functional stability of retinal ganglion cells after degeneration-induced changes in synaptic input. J Neurosci. 2008; 28:6526–6536. PMID: 18562624.
Article15. Ryu SB, Ye JH, Lee JS, Goo YS, Kim CH, Kim KH. Electrically-evoked neural activities of rd1 mice retinal ganglion cells by repetitive pulse stimulation. Korean J Physiol Pharmacol. 2009; 13:443–448. PMID: 20054490.
Article16. Ryu SB, Ye JH, Goo YS, Kim CH, Kim KH. Temporal response properties of retinal ganglion cells in rd1 mice evoked by amplitude-modulated electrical pulse trains. Invest Ophthalmol Vis Sci. 2010; 51:6762–6769. PMID: 20671284.17. Goo YS, Ye JH, Lee S, Nam Y, Ryu SB, Kim KH. Retinal ganglion cell responses to voltage and current stimulation in wild-type and rd1 mouse retinas. J Neural Eng. 2011; 8:035003. PMID: 21593549.18. Della Santina L, Bouly M, Asta A, Demontis GC, Cervetto L, Gargini C. Effect of HCN channel inhibition on retinal morphology and function in normal and dystrophic rodents. Invest Ophthalmol Vis Sci. 2010; 51:1016–1023. PMID: 19741244.19. Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007; 500:222–238. PMID: 17111372.
Article20. Munk MH, Neuenschwander S. High-frequency oscillations (20 to 120 Hz) and their role in visual processing. J Clin Neurophysiol. 2000; 17:341–360. PMID: 11012039.
Article21. Goo YS, Ahn KN, Song YJ, Ryu SB, Kim KH. Comparison of basal oscillatory rhythm of retinal activities in rd1 and rd10 mice. Conf Proc IEEE Eng Med Biol Soc. 2011. p. 1093–1096. PMID: 22254504.
Article22. Stett A, Barth W, Weiss S, Haemmerle H, Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vision Res. 2000; 40:1785–1795. PMID: 10814763.
Article23. Hayes MH. Statistical digital signal processing and modeling. 1996. 1st ed. New York: John Wiley & Sons.24. Stasheff SF, Shankar M, Andrews MP. Developmental time course distinguishes changes in spontaneous and light-evoked retinal ganglion cell activity in rd1 and rd10 mice. J Neurophysiol. 2011; 105:3002–3009. PMID: 21389300.
Article25. Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978; 17:489–498. PMID: 659071.26. Strettoi E, Porciatti V, Falsini B, Pignatelli V, Rossi C. Morphological and functional abnormalities in the inner retina of the rd/rd mouse. J Neurosci. 2002; 22:5492–5504. PMID: 12097501.
Article27. Phillips MJ, Otteson DC, Sherry DM. Progression of neuronal and synaptic remodeling in the rd10 mouse model of retinitis pigmentosa. J Comp Neurol. 2010; 518:2071–2089. PMID: 20394059.28. Koepsell K, Wang X, Hirsch JA, Sommer FT. Exploring the function of neural oscillations in early sensory systems. Front Neurosci. 2010; 4:53. PMID: 20582272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electrophysiological and Histologic Evaluation of the Time Course of Retinal Degeneration in the rd10 Mouse Model of Retinitis Pigmentosa
- Comparison of Retinal Ganglion Cell Responses to Different Voltage Stimulation Parameters in Normal and rd1 Mouse Retina
- A Clinical Study on the Fellow Eyes in Unilateral Retinal Detahment
- Etiological Analysis of Non Traumatic, Non Diabetic Spontaneous Vitreous Hemorrhage Using Vitrectomy
- Activation of Caspase-3 During Photoreceptor Degeneration in rd Mouse Retina