Korean J Physiol Pharmacol.
2011 Apr;15(2):115-122. 10.4196/kjpp.2011.15.2.115.
Matrix Metalloproteinase Inhibitors Attenuate Neuroinflammation Following Focal Cerebral Ischemia in Mice
- Affiliations
-
- 1Department of Pharmacology, and Medical Research Center for Ischemic Tissue Regeneration, Pusan National University School of Medicine, Yangsan 626-870, Korea. wonslee@pusan.ac.kr
- KMID: 2285416
- DOI: http://doi.org/10.4196/kjpp.2011.15.2.115
Abstract
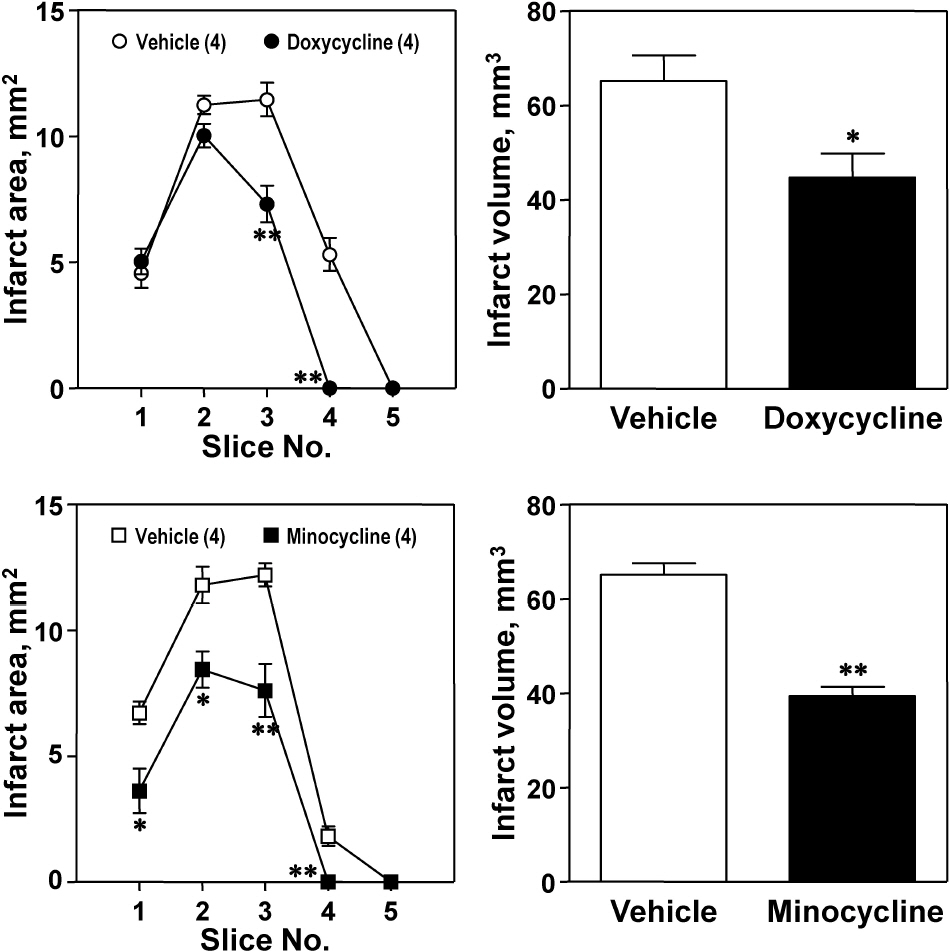
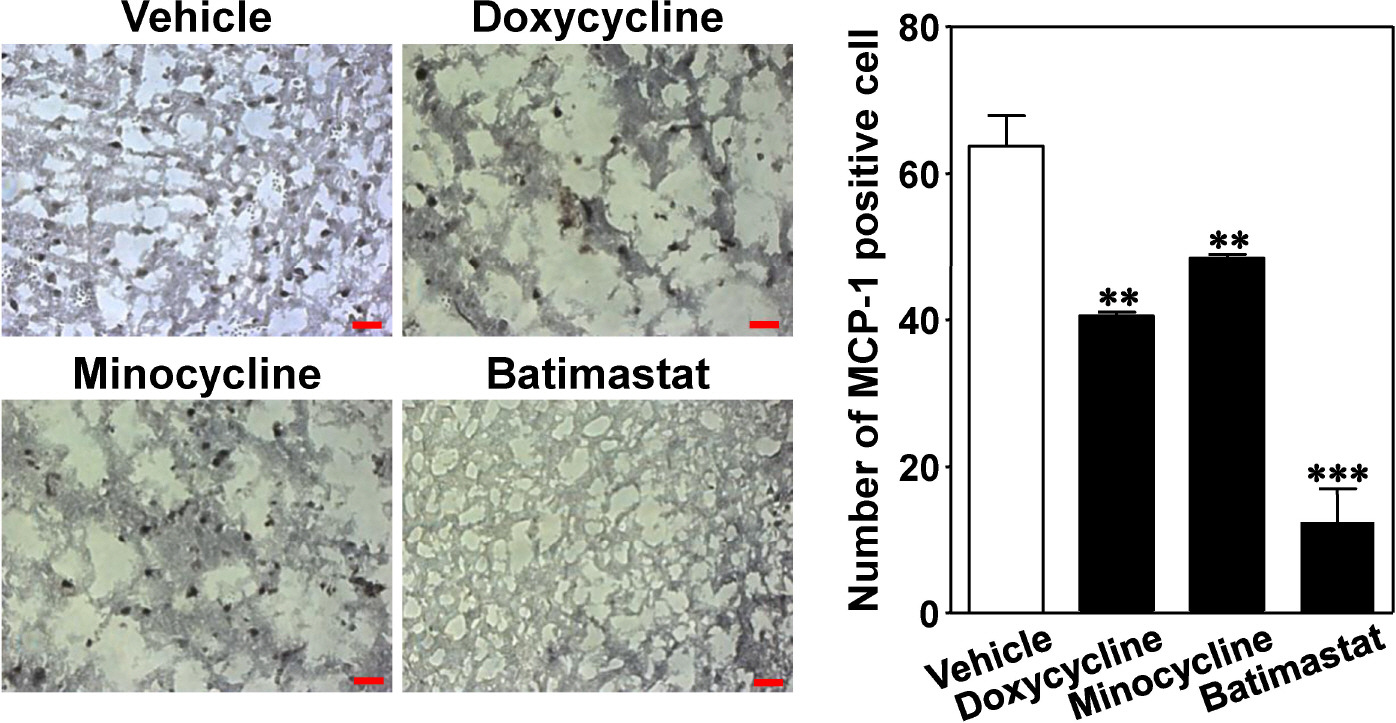
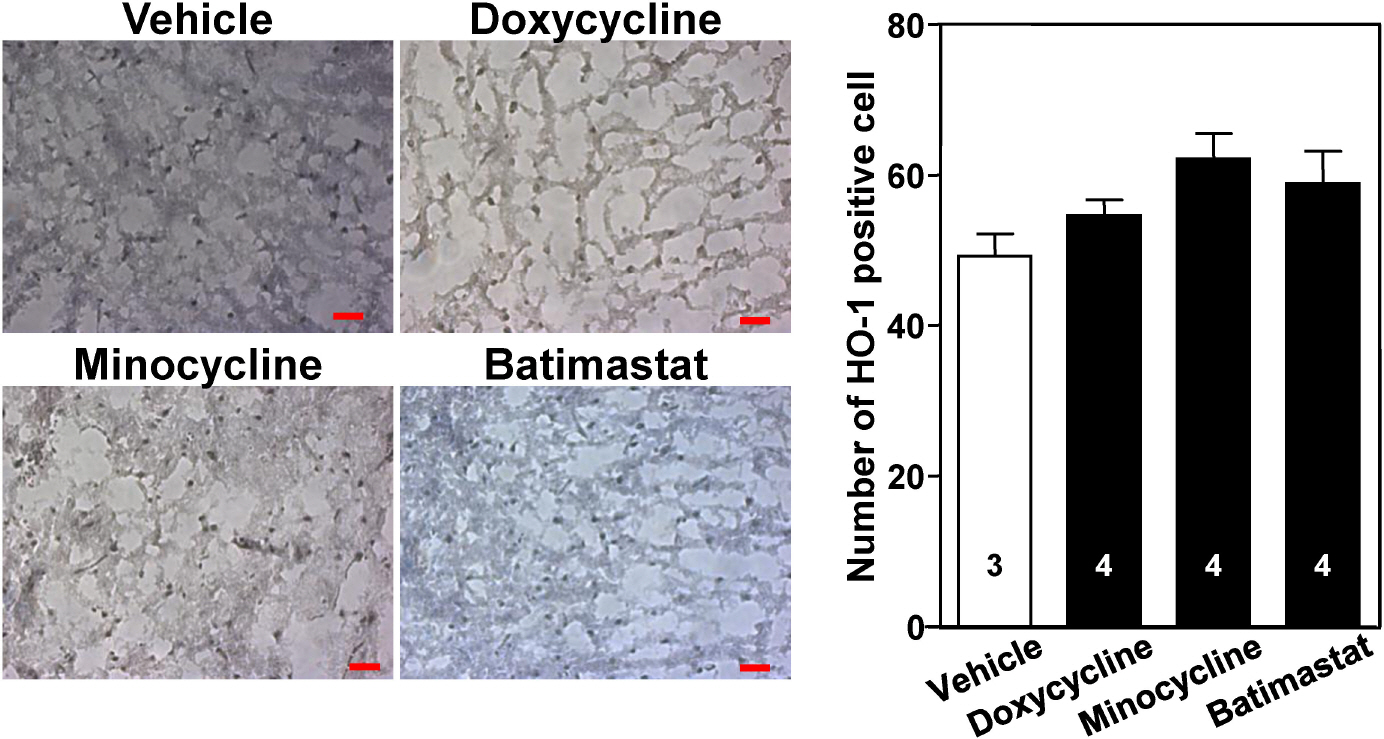
- The aim of this study was to investigate whether matrix metalloproteinase (MMP) inhibitors attenuate neuroinflammation in an ischemic brain following photothrombotic cortical ischemia in mice. Male C57BL/6 mice were anesthetized, and Rose Bengal was systemically administered. Permanent focal ischemia was induced in the medial frontal and somatosensory cortices by irradiating the skull with cold white light. MMP inhibitors, such as doxycycline, minocycline, and batimastat, significantly reduced the cerebral infarct size, and the expressions of monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-alpha), and indoleamine 2,3-dioxygenase (IDO). However, they had no effect on the expressions of heme oxygenase-1 and neuroglobin in the ischemic cortex. These results suggest that MMP inhibitors attenuate ischemic brain injury by decreasing the expression levels of MCP-1, TNF-alpha, and IDO, thereby providing a therapeutic benefit against cerebral ischemia.
Keyword
MeSH Terms
-
Animals
Brain
Brain Injuries
Brain Ischemia
Chemokine CCL2
Cold Temperature
Doxycycline
Globins
Heme Oxygenase-1
Humans
Indoleamine-Pyrrole 2,3,-Dioxygenase
Ischemia
Light
Male
Matrix Metalloproteinase Inhibitors
Mice
Minocycline
Nerve Tissue Proteins
Phenylalanine
Rose Bengal
Skull
Thiophenes
Tumor Necrosis Factor-alpha
Chemokine CCL2
Doxycycline
Globins
Heme Oxygenase-1
Indoleamine-Pyrrole 2,3,-Dioxygenase
Matrix Metalloproteinase Inhibitors
Minocycline
Nerve Tissue Proteins
Phenylalanine
Rose Bengal
Thiophenes
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Caffeic acid phenethyl ester protects against photothrombotic cortical ischemic injury in mice
Sun Ae Hwang, Chi Dae Kim, Won Suk Lee
Korean J Physiol Pharmacol. 2018;22(1):101-110. doi: 10.4196/kjpp.2018.22.1.101.
Reference
-
References
1. Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010; 24:708–723.
Article2. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009; 7:97.
Article3. Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002; 39:279–291.
Article4. Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998; 18:1163–1172.
Article5. Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000; 20:1681–1689.
Article6. Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001; 21:1393–1400.
Article7. Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998; 29:1020–1030.8. Wang X, Yue TL, Barone FC, Feuerstein GZ. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke. 1995; 26:661–665.
Article9. Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003; 23:748–755.
Article10. Minami M, Satoh M. Chemokines and their receptors in the brain: pathophysiological roles in ischemic brain injury. Life Sci. 2003; 74:321–327.
Article11. Conductier G, Blondeau N, Guyon A, Nahon JL, Rovère C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010; 224:93–100.
Article12. Sriram K, O'Callaghan JP. Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol. 2007; 2:140–153.13. Brenner DA, O'Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989; 337:661–663.14. Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993; 296:803–809.15. Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res. 1998; 801:1–8.
Article16. Yang GY, Gong C, Qin Z, Liu XH, Lorris Betz A. Tumor necrosis factor alpha expression produces increased blood-brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1999; 69:135–143.
Article17. Saito K, Nowak TS Jr, Suyama K, Quearry BJ, Saito M, Crowley JS, Markey SP, Heyes MP. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J Neurochem. 1993; 61:2061–2070.
Article18. Heyes MP, Saito K, Jacobowitz D, Markey SP, Takikawa O, Vickers JH. Poliovirus induces indoleamine-2,3-dioxygenase and quinolinic acid synthesis in macaque brain. FASEB J. 1992; 6:2977–2989.
Article19. Heyes MP, Saito K, Devinsky O, Nadi NS. Kynurenine pathway metabolites in cerebrospinal fluid and serum in complex partial seizures. Epilepsia. 1994; 35:251–257.
Article20. Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, Hunt NH. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998; 152:611–619.21. Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl) thiazol-2yl]-benzenesulfonamide (Ro 61–8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab. 1999; 19:771–777.22. Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 1991; 12:185–188.
Article23. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997; 37:517–554.24. Geddes JW, Pettigrew LC, Holtz ML, Craddock SD, Maines MD. Permanent focal and transient global cerebral ischemia increase glial and neuronal expression of heme oxygenase-1, but not heme oxygenase-2, protein in rat brain. Neurosci Lett. 1996; 210:205–208.
Article25. Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999; 72:1187–1203.
Article26. Fu R, Zhao ZQ, Zhao HY, Zhao JS, Zhu XL. Expression of heme oxygenase-1 protein and messenger RNA in permanent cerebral ischemia in rats. Neurol Res. 2006; 28:38–45.
Article27. Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000; 407:520–523.
Article28. Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001; 98:15306–15311.
Article29. Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003; 100:3497–3500.
Article30. Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. J Neurosci Methods. 2002; 117:43–49.
Article31. Shin TK, Kang MS, Lee HY, Seo MS, Kim SG, Kim CD, Lee WS. Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol. 2009; 13:257–263.
Article32. Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986; 17:1304–1308.
Article33. Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007; 27:1213–1224.
Article34. Muir KW, Tyrrell P, Sattar N, Warburton E. Inflammation and ischaemic stroke. Curr Opin Neurol. 2007; 20:334–342.
Article35. del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003; 23:879–894.
Article36. Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004; 35:998–1004.
Article37. Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol. 2002; 37:375–536.
Article38. Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005; 50:329–339.
Article39. Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. 2003; 34:2165–2170.40. Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006; 37:1399–1406.
Article41. Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995; 26:2120–2126.
Article42. Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996; 739:88–96.
Article43. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007; 38:1044–1049.44. Thornton P, Pinteaux E, Allan SM, Rothwell NJ. Matrix metalloproteinase-9 and urokinase plasminogen activator mediate interleukin-1-induced neurotoxicity. Mol Cell Neurosci. 2008; 37:135–142.
Article45. McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008; 28:9451–9462.
Article46. Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998; 12:12–26.
Article47. Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs. 1997; 15:61–75.48. Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005; 25:460–467.
Article49. Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998; 392:565–568.
Article50. Furie MB, Randolph GJ. Chemokines and tissue injury. Am J Pathol. 1995; 146:1287–1301.51. Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995; 56:127–134.
Article52. Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001; 902:171–177.
Article53. Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002; 22:308–317.
Article54. Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007; 38:1345–1353.
Article55. Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009; 276:13–26.
Article56. Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997; 28:1233–1244.57. Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994; 370:555–557.58. Dittmar M, Kiourkenidis G, Horn M, Bollwein S, Bernhardt G. Cerebral ischemia, matrix metalloproteinases, and TNF-alpha: MMP inhibitors may act not exclusively by reducing MMP activity. Stroke. 2004; 35:e338–340.
Article59. Mayhan WG. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002; 927:144–152.60. Németh H, Toldi J, Vécsei L. Kynurenines, Parkinson's disease and other neurodegenerative disorders: preclinical and clinical studies. J Neural Transm Suppl. 2006; 70:285–304.
Article61. Mackay GM, Forrest CM, Stoy N, Christofides J, Egerton M, Stone TW, Darlington LG. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2006; 13:30–42.
Article62. Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000; 20:53–65.
Article63. Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000; 31:1735–1743.
Article64. Gehrmann J, Bonnekoh P, Miyazawa T, Hossmann KA, Kreutzberg GW. Immunocytochemical study of an early microglial activation in ischemia. J Cereb Blood Flow Metab. 1992; 12:257–269.
Article65. Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000; 279:L1029–1037.
Article66. Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002; 234–235:249–263.
Article67. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997; 272:5375–5381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of (-)-Epigallocatechin-3-gallate on Brain Infarction and the Activity Change of Matrix Metalloproteinase-9 Induced by Middle Cerebral Artery Occlusion in Mice
- Fluoxetine and Sertraline Attenuate Postischemic Brain Injury in Mice
- Matrix Metalloproteinases in Cerebral Ischemia
- The Differential expression of matrix metalloproteinase and their tissue inhibitors in myometrium and leiomyoma
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients