Korean J Physiol Pharmacol.
2010 Jun;14(3):157-161. 10.4196/kjpp.2010.14.3.157.
Loss of hsp70.1 Decreases Functional Motor Recovery after Spinal Cord Injury in Mice
- Affiliations
-
- 1Department of Dental Anesthesiology and Dental Research Institute, Seoul National University School of Dentistry, Seoul 110-744, Korea.
- 2Department of Physiology, Korea University College of Medicine, Seoul 136-705, Korea.
- 3Department of Physical Therapy, Korea University College of Health Science, Seoul 136-703, Korea. junokim@korea.ac.kr
- 4Divisions of Radiation Cancer Research and Korea Institute of Radiological and Medical Sciences, Seoul 139-706, Korea.
- KMID: 2285397
- DOI: http://doi.org/10.4196/kjpp.2010.14.3.157
Abstract
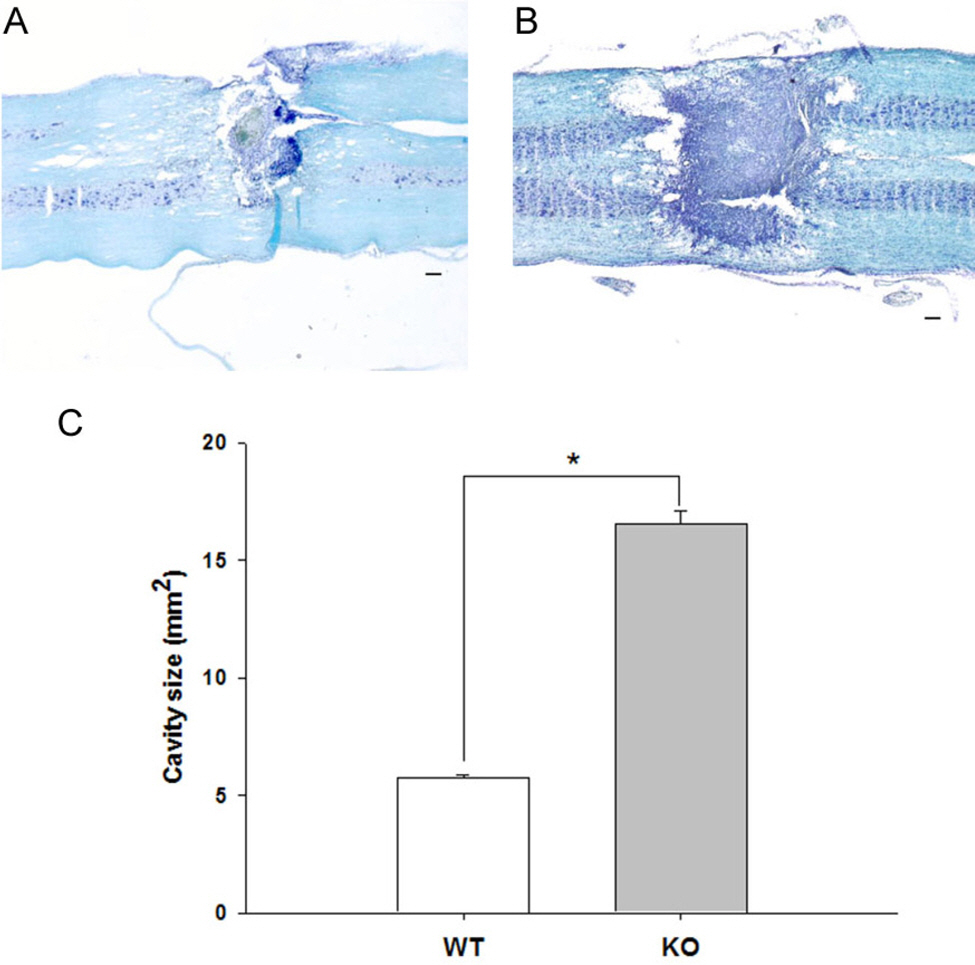
- Heat shock proteins (HSPs) are specifically induced by various forms of stress. Hsp70.1, a member of the hsp70 family is known to play an important role in cytoprotection from stressful insults. However, the functional role of Hsp70 in motor function after spinal cord injury (SCI) is still unclear. To study the role of hsp70.1 in motor recovery following SCI, we assessed locomotor function in hsp70.1 knockout (KO) mice and their wild-type (WT) mice via the Basso, Beattie and Bresnahan (BBB) locomotor rating scale, before and after spinal hemisection at T13 level. We also examined lesion size in the spinal cord using Luxol fast blue/cresyl violet staining. One day after injury, KO and WT mice showed no significant difference in the motor function due to complete paralysis following spinal hemisection. However, when it compared to WT mice, KO mice had significantly delayed and decreased functional outcomes from 4 days up to 21 days after SCI. KO mice also showed significantly greater lesion size in the spinal cord than WT mice showed at 21 days after spinal hemisection. These results suggest that Hsp70 has a protective effect against traumatic SCI and the manipulation of the hsp70.1 gene may help improve the recovery of motor function, thereby enhancing neuroprotection after SCI.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004; 4:451–464.
Article2. Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998; 56:341–358.
Article3. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991; 75:15–26.
Article4. Bethea JR. Spinal cord injury-induced inflammation: a dual-edged sword. Neural Plast Regen. 2000; 128:33–42.
Article5. Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004; 24:4043–4051.
Article6. Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992; 76:13–22.
Article7. Kunz S, Tegeder I, Coste O, Marian C, Pfenninger A, Corvey C, Karas M, Geisslinger G, Niederberger E. Comparative proteomic analysis of the rat spinal cord in inflammatory and neuropathic pain models. Neurosci Lett. 2005; 381:289–293.
Article8. Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996; 381:571–580.
Article9. Abe T, Konishi T, Hirano T, Kasai H, Shimizu K, Kashimura M, Higashi K. Possible correlation between DNA damage induced by hydrogen peroxide and translocation of heat shock 70 protein into the nucleus. Biochem Biophys Res Comm. 1995; 206:548–555.
Article10. Fukamachi Y, Karasaki Y, Sugiura T, Itoh H, Abe T, Yamamura K, Higashi K. Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio. Biochem Biophys Res Comm. 1998; 246:364–369.
Article11. Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes – a paradigm for inducible genomic responses. J Biol Chem. 1992; 267:21987–21990.12. Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Phsyio Lung Cell Mol Physiol. 1999; 277:L1026–L1033.13. Kabakov AE, Gabai VL. Heat shock induced accumulation of 70 kDa stress protein (HSP70) can protect ATP depleted tumor cells from necrosis. Exp Cell Res. 1995; 217:15–21.14. Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70kDa heat stress protein transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995; 95:1446–1456.15. Turner CP, Panter SS, Sharp FR. Anti-oxidants prevent focal rat brain injury as assessed by induction of heat shock proteins (HSP70, HO-1/HSP32, HSP47) following subarachnoid injections of lysed blood. Mol Brain Res. 1999; 65:87–102.
Article16. Plumier JCL, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997; 2:162–167.
Article17. Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000; 47:782–791.
Article18. Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG. Differential neuroprotection from human heat shock protein 70 3roverexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol. 2001; 170:129–139.19. Awad H, Suntres Z, Heijmans J, Smeak D, Bergdall-Costell V, Christofi FL, Magro C, Oglesbee M. Intracellular and extracellular expression of the major inducible 70 kDa heat shock protein in experimental ischemia-reperfusion injury of the spinal cord. Exp Neurol. 2008; 212:275–284.20. Brown IR. Heat shock proteins and protection of the nervous system. Stress Resp Biol Med. 2007; 1113:147–158.
Article21. Kikuchi S, Shinpo K, Takeuchi M, Tsuji S, Yabe I, Niino M, Tashiro K. Effect of geranylgeranylaceton on cellular damage induced by proteasome inhibition in cultured spinal neurons. J Neurosci Res. 2002; 69:373–381.
Article22. Manabe Y, Wang JM, Murakami T, Warita H, Hayashi T, Shoji M, Abe K. Expressions of nitrotyrosine and TUNEL immunore-activities in cultured rat spinal cord neurons after exposure to glutamate, nitric oxide, or peroxynitrite. J Neurosci Res. 2001; 65:371–377.
Article23. Cizkova D, Carmel JB, Yamamoto K, Kakinohana O, Sun D, Hart RP, Marsala M. Characterization of spinal HSP72 induction and development of ischemic tolerance after spinal ischemia in rats. Exp Neurol. 2004; 185:97–108.
Article24. Perdrizet GA, Lena CJ, Shapiro DS, Rewinski MJ. Preoperative stress conditioning prevents paralysis after experimental aortic surgery: Increased heat shock protein content is associated with ischemic tolerance of the spinal cord. J Thorac Cardiovasc Surg. 2002; 124:162–170.
Article25. Willoughby DS, Priest JW, Nelson M. Expression of the stress proteins, ubiquitin, heat shock protein 72, and myofibrillar protein content after 12 weeks of leg cycling in persons with spinal cord injury. Arch Phys Med Rehabil. 2002; 83:649–654.
Article26. Sasara T, Cizkova D, Mestril R, Galik J, Sugahara K, Marsala M. Spinal heat shock protein (70) expression: effect of spinal ischemia, hyperthermia (42 degrees C)/hypothermia (27 degrees C), NMDA receptor activation and potassium evoked depolarization on the induction. Neuroschem Intern. 2004; 44:53–64.27. Kalmar B, Burnstock G, Vrbova G, Urbanics R, Csermely P, Greensmith L. Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats. Exp Neurol. 2002; 176:87–97.
Article28. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21.
Article29. Kim J, Yoon YW, Hong SK, Na HS. Cold and mechanical allodynia in both hindpaws and tail following thoracic spinal cord hemisection in rats: Time courses and their correlates. Neurosci Lett. 2003; 343:200–204.
Article30. Tanabe M, Ono K, Honda M, Ono H. Gabapentin and pregabalin ameliorate mechanical hypersensitivity after spinal cord injury in mice. Eur J Pharmacol. 2009; 609:65–68.
Article31. Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007; 320:1002–1012.32. Shin YC, Choi KY, Kim WG. Cyclosporin A has a protective effect with induced upregulation of Hsp70 and nNOS on severe spinal cord ischemic injury in rabbits. J Invest Surg. 2007; 20:113–120.
Article33. Matsumoto M, Ohtake K, Wakamatsu H, Oka S, Kiyoshima T, Nakakimura K, Sakabe T. The time course of acquisition of ischemic tolerance and induction of heat shock protein 70 after a brief period of ischemia in the spinal cord in rabbits. Anesth Anal. 2001; 92:418–423.
Article34. Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol. 2001; 171:153–169.
Article35. Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004; 24:2182–2190.
Article36. Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006; 84:1064–1075.
Article37. Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005; 82:283–293.
Article38. Song GQ, Cechvala C, Resnick DK, Dempsey RJ, Rao VLR. GeneChip((R)) analysis after acute spinal cord injury in rat. J Neurochem. 2001; 79:804–815.39. Hecker JG, Sundram H, Zou S, Praestgaard A, Bavaria JE, Ramchandren S, McGarvey M. Heat shock proteins HSP70 and HSP27 in the cerebral spinal fluid of patients undergoing thoracic aneurysm repair correlate with the probability of postoperative paralysis. Cell Stress Chaperones. 2008; 13:435–446.
Article40. Lee JR, Han SM, Leem JG, Hwang SJ. Effects of intrathecal bupivacaine in conjunction with hypothermia on neuronal protection against transient spinal cord ischemia in rats. Acta Anaesthesiol Scand. 2007; 51:60–67.
Article41. Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW. Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets. 2007; 6:91–100.42. Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: A critical component for motoneuron survival. J Neurosci. 2005; 25:9735–9745.
Article43. Sharma HS, Gordh T, Wiklund L, Mohanty S, Sjoquist PO. Spinal cord injury induced heat shock protein expression is reduced by an antioxidant compound H-290/51. An experimental study using light and electron microscopy in the rat. J Neural Transm. 2006; 113:521–536.
Article44. Tachibana T, Noguchi K, Ruda MA. Analysis of gene expression following spinal cord injury in rat using complementary DNA microarray. Neurosci Lett. 2002; 327:133–137.
Article45. Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005; 11:1088–1095.
Article46. Walter L, Rauh F, Gunther E. Comparative analysis of the 3 major histocompatiblity complex linked heat shock protein 70 (HSP70) genes of the rat. Immunogenetics. 1994; 40:325–330.47. Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004; 35:2195–2199.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Time-course of Neurologic Recovery in Traumatic Spinal Cord Injury
- Effects of Functional Magnetic Stimulation on the Functional Recovery in a Rat Model of Spinal Cord Injury
- Motor Evoked Potentials and Recovery of Motor Function in Spinal Cord Injuried Rats
- Current Concept and Future of the Management of Spinal Cord Injury: A Systematic Review
- Functional Recovery of Patients with Traumatic Central Cord Syndrome